Abstract
Ginseng has been used as a popular herbal medicine in East Asia for at least two millennia. However, 20(R)-ginseng saponins, one class of important rare ginsenosides, are rare in natural products. 20(R)-ginseng saponins are generally prepared by chemical epimerization and microbial transformation from 20(S)-isomers. The C20 configuration of 20(R)-ginseng saponins are usually determined by 13C NMR and X-ray single-crystal diffraction. 20(R)-ginseng saponins have antitumor, antioxidative, antifatigue, neuroprotective, and osteoclastogenesis inhibitory effects, among others. Owing to the chemical structure and pharmacological and stereoselective properties, 20(R)-ginseng saponins have attracted a great deal of attention in recent years. In this study, the discovery, identification, chemical epimerization, microbial transformation, pharmacological activities, and metabolism of 20(R)-ginseng saponins are summarized.
Keywords: 20(R)-ginseng saponin, Epimerization, Identification, Metabolism, Pharmacological activity
1. Introduction
Ginseng, a functional food and health-enhancing supplement, is a global herb and has been shown to have extensive range of pharmacological effects on cognition and blood circulation, as well as antitumor, antioxidative, and antifatigue effects, among others [1]. Many of its components, such as ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids, have been separated and characterized. Ginsenosides are common pharmacological components in ginseng herbs, named after the originating countries, for example Panax ginseng, P. japonicas, P. notoginseng, P. quinquefolius, and P. vietnamensis [2]. In 1854, Garriques [3] conducted a chemical study on ginseng and separated a saponin fraction from P. quinquifolium L. for the first time. Despite these findings, the saponin fraction, which contained so-called ginsenosides, was not isolated and identified again until 1963 [4].
So far, more than 100 different ginsenosides with different pharmacological activities have been separated and identified from the root of red ginseng (P. ginseng). Red ginseng is prepared by steaming fresh ginseng at 90–100°C for a reasonable time and then dried until the moisture content is less than 15% [5]. The amounts of some ginsenosides, such as Rb1, Rc, Rb2 and Rd, in red ginseng are higher than those in fresh ginseng [6], and they have biological functions including the amelioration of various disease symptoms via antioxidant mechanisms in cells and animals (especially in rodents) [7]. Meanwhile, 20(R)-ginseng saponins have been found in P. ginseng. Kitagawa et al [9] isolated 20(R)-Rh1 (4) and 20(R)-Rg2 (5) as characteristic constituents of Ginseng Radix Rubra in 1983. 20(R)-Rh2 (1) was first isolated from P. ginseng leaves and identified in 1987 by Chen et al [10].
The C20 configuration confirmation of ginsenosides had become the focus of this research field because they might play a vital role in pharmacological activities. Compared with other ginsenosides, 20(R)-ginseng saponins have better pharmacological activities [8], such as antitumor [11], [12], antioxidative [13], antifatigue [14], neuroprotective [15], and osteoclastogenesis inhibitory effects [16]. Li et al [13] found that 20(R)-Rg3 (2) exhibited better antioxidative activity in vitro than 20(S)-Rg3. In addition, 20(R)-Rh2 (1), but not 20(S)-Rh2, is a selective osteoclastogenesis inhibitor without any cytotoxicity [16]. Thus, chemical structure, pharmacological action, and metabolism of 20(R)-ginseng saponins have aroused interest. In this article, the discovery, structure identification, chemical epimerization, microbial transformation, pharmacological activities, and metabolism of 20(R)-ginseng saponins are summarized.
2. Discovery of 20(R)-ginseng saponins
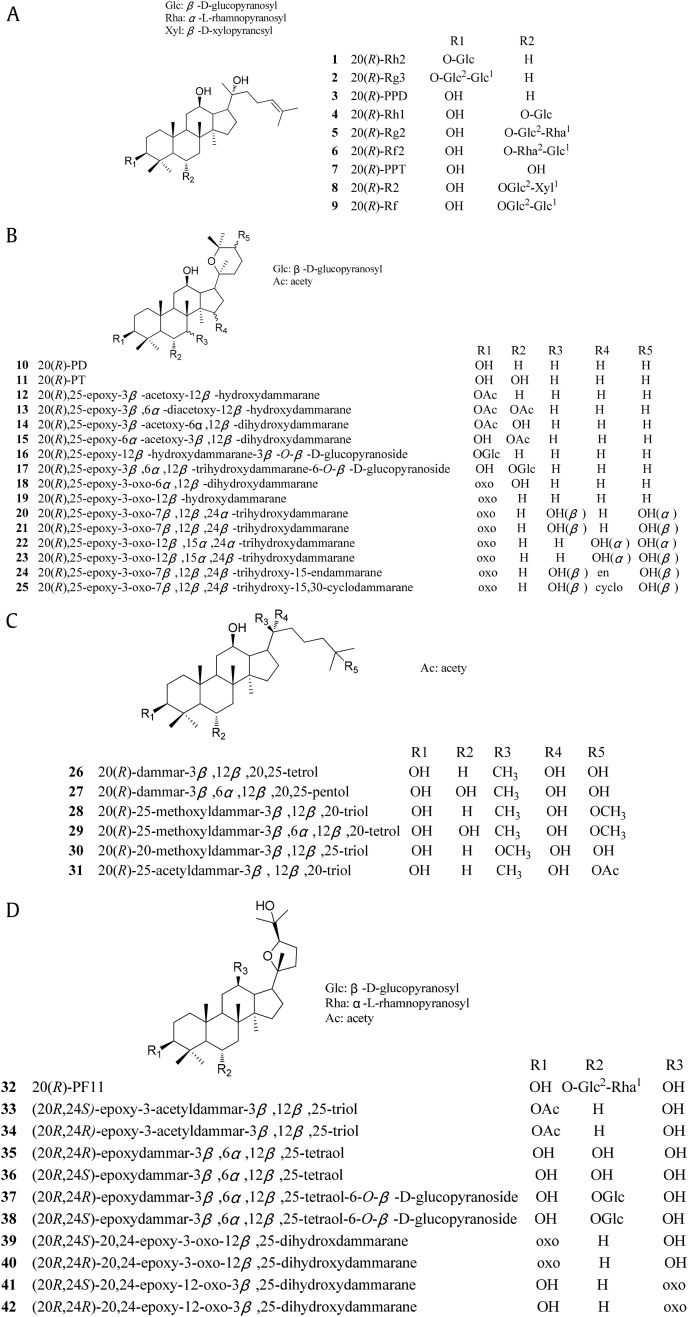
20(R)-dammarane-type saponins are generally classified into protopanaxadiol (PPD) type and protopanaxatriol (PPT) type. The PPD-type saponin involves the attachment of saccharide(s) to C3 and/or C20, and the PPT-type saponin involves the attachment of saccharide(s) to C3, C6, and/or C20. The ocotillol-type saponin is a tetracyclic triterpenoid, containing a tetrahydrofuran ring [17]. The structures of all 20(R)-ginseng saponins are shown in Fig. 1.
Fig. 1.
The structures of 20(R)-ginseng saponins. (A) Protopanaxadiol (PPD)- and protopanaxatriol (PPT)-type saponins; (B) Panaxadiol (PD)-type, panaxatriol (PT)-type, and modified saponins; (C) Modified PPD- and PPT-type saponins; (D) Ocotillol-type and modified saponins.
2.1. 20(R)-PPD-type saponins
20(R)-Rh2 (1, 40 mg) was first isolated from the stems and leaves of P. ginseng Meyer (100 kg) by Chen et al [10]. It [m.p. 288–290°C; +21.1 (c 0.61, MeOH)] was identified by 1H NMR, 13C NMR, and MS and compared with the reported 20(S)-Rh2 13C NMR data [18], [19], [20].
20(R)-Rg3 (2, 1.5 g, m.p. 298–300°C) and 20(R)-PPD (3, 8 mg, m.p. 243–245°C) were isolated from the root, stems, and leaves of P. ginseng (5 kg) by incision during the steaming process [21], [22]. They found that Compound 3 was generated by steaming fresh ginseng, and the content was higher than that in red ginseng. The content was also higher in fine roots than that in main roots. These structures were identified by 1H NMR, 13C NMR, and electrospray ionization mass spectrometry (ESI-MS) [18], [19], [20], [23].
2.2. 20(R)-PPT-type saponins
20(R)-Rh1 (4, 900 mg, m.p. 217–219°C) was first separated and purified from P. ginseng Meyer (5 kg) by silica gel column chromatography (CHCl3/MeOH/H2O, 6:1:0.1) and solid-phase extraction high performance liquid chromatography (SP-HPLC, MeOH/H2O, 70:30) [21]. Wang et al [24] obtained Compound 4 from the root of P. notoginseng (Burk.) F. H. Chen (Araliaceae) by steaming and baking. Compound 4 (35 mg) and 20(R)-Rg2 (5, 16 mg) from Gynostemma yixingense (100 g) were subjected to silica gel column chromatography (CHCl3/MeOH, 20:1 to 1:1) [20]. Their structures were determined by spectroscopic analyses, including 1D-NMR, 2D-NMR, and ESI-MS [18], [19].
20(R)-Rf2 (6, 6 mg) and 20(R)-PPT (7, 120 mg, m.p. 259–261°C) were isolated from the stems and leaves of P. ginseng (5 kg) and purified by silica gel column chromatography (CHCl3/MeOH/H2O, 3:1:0.1) by Xu et al [25] and Yang et al [21]. Their structures were identified by IR, field desorption-mass spectrometry (FD-MS), and comparison with the 20(S)-isomer's 13C NMR data [26], [27].
2.3. Ocotillol-type saponins
20(R)-PF11 (32, 11 mg) was obtained from American red ginseng (2.5 kg), and novel structures will likely continue to be reported with the aid of modern and more sensitive characterization techniques. Its structure was elucidated as (20R,24R)-dammar-20, 24-epoxy-3α, 6α,12β, 25-tetraol [28], [29].
3. Identification of 20(R)-ginseng saponins
3.1. 13C NMR analysis
NMR plays a vital role in the structural elucidation of 20(R/S)-ginseng saponins. The obvious identifiers are the chemical shift values of C20 and its contiguous C17, C21, and C22.
Asakawa et al [30] found that the chemical shift values of C17, C21, and C22 of 20(S)-ginseng saponins were ca. 55, 27, and 35 ppm, respectively, distinctively different from those of the 20(R)-isomers (ca. 50, 22, and 43 ppm). In addition, changes in chemical shifts between the S form and R form at C17, C21, and C22 in 13C NMR spectra were approximately ▵δ (δR-δS) −4.1 ± 0.1, −4.3 ± 0.1, and +7.4 ± 0.1 ppm, respectively [31] (Table 1).
Table 1.
Chemical shift of C-17, C-20, C-21, and C-22 in 20(R)-ginseng saponins (in pyridine-d5)
| Ingredient name | C-17 | C-20 | C-21 | C-22 | Refs |
|---|---|---|---|---|---|
| 20(R)-Rh2 | 52.2 | 73.4 | 23.0 | 43.7 | [9] |
| 20(S)-Rh2 | 54.8 | 72.9 | 26.9 | 35.2 | |
| ▵δ20(R)-20(S) | −2.6 | +0.5 | −3.9 | +8.5 | |
| 20(R)-Rg3 | 50.7 | 73.0 | 22.8 | 43.3 | [9] |
| 20(S)-Rg3 | 54.8 | 73.0 | 27.1 | 35.9 | |
| ▵δ20(R)-20(S) | −4.1 | ±0 | −4.3 | +2.6 | |
| 20(R)-PPD | 50.7 | 73.0 | 22.6 | 43.2 | [9] |
| 20(S)-PPD | 54.9 | 73.0 | 25.8 | 35.3 | |
| ▵δ20(R)-20(S) | −4.2 | ±0 | −3.2 | +7.9 | |
| 20(R)-Rh1 | 50.6 | 73.0 | 23.0 | 43.3 | [9] |
| 20(S)-Rh1 | 54.6 | 73.0 | 26.9 | 35.9 | |
| ▵δ20(R)-20(S) | −4.0 | ±0 | −3.9 | +7.4 | |
| 20(R)-Rg2 | 49.7 | 73.0 | 22.7 | 43.2 | [15], [16] |
| 20(S)-Rg2 | 54.6 | 72.9 | 26.9 | 35.7 | |
| ▵δ20(R)-20(S) | −4.9 | +0.1 | −4.2 | +7.5 | |
| 20(R)-Rf2 | 51.7 | 73.4 | 22.5 | 43.6 | [9] |
| 20(S)-Rf2 | 54.7 | 72.5 | 27.2 | 36.4 | |
| ▵δ20(R)-20(S) | −3.0 | +0.9 | −4.7 | +7.2 | |
| 20(R)-PPT | 50.7 | 73.0 | 22.6 | 43.3 | [9] |
| 20(S)-PPT | 54.8 | 73.0 | 27.1 | 35.9 | |
| ▵δ20(R)-20(S) | −4.1 | ±0 | −4.5 | +7.4 | |
| 20(R)-PF11 | 50.5 | 86.3 | 19.3 | 38.2 | [25], [26] |
| 20(S)-PF11 | 48.8 | 87.3 | 28.8 | 31.5 | |
| ▵δ20(R)-20(S) | +1.7 | −1.0 | −9.5 | +6.7 |
PPD, protopanaxadiol; PPT, protopanaxatriol.
The observed chemical shift values of each compound are within the ranges mentioned previously, which permits the assignment of the C20 configuration as S form or R form.
3.2. Single-crystal X-ray diffraction
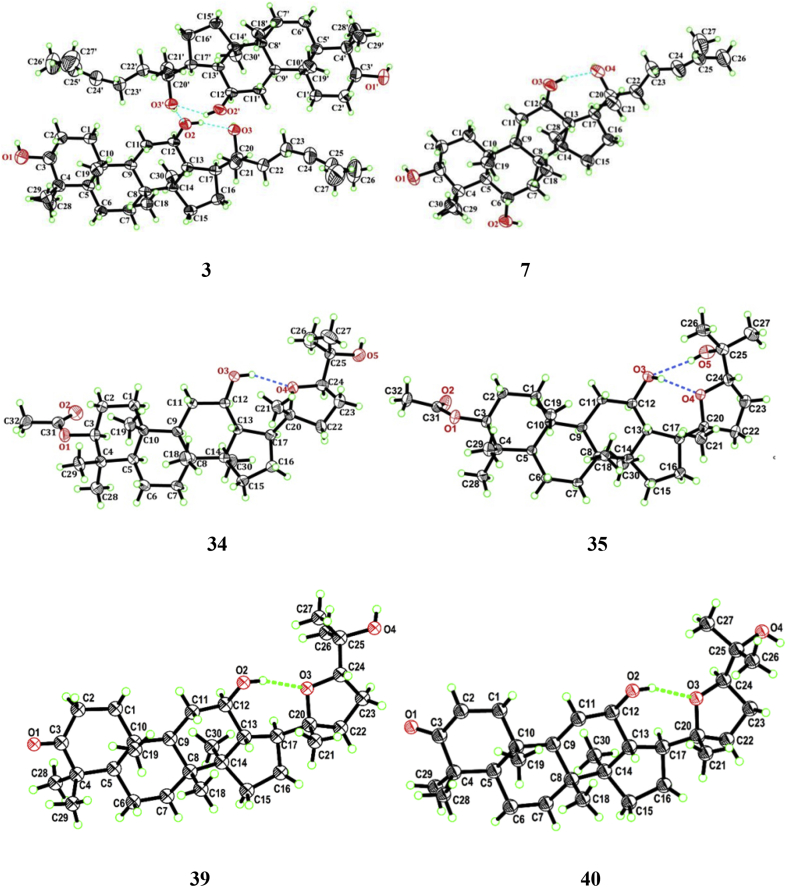
The C20 configuration of 20(R/S)-ginseng saponins could also be determined by single-crystal X-ray diffraction analysis. In our previous study, Compounds 3 and 7 were prepared from P. quinquefolium L. with citric acid and sodium hydrate in glycerol, successively. (20R,24S)-epoxy-3-acetyldammar-3β,12β,25-triol (33), (20R,24R)-epoxy-3-acetyldammar-3β,12β,25-triol (34), (20R,24S)-20,24-epoxy-3-oxo-12β,25-dihydroxdammarane (39), and (20R,24R)-20,24-epoxy-3-oxo-12β,25-dihydroxy-dammarane (40) have been synthesized from Compound 3. Their structures were elucidated by spectral studies, and the configurations were confirmed by single-crystal X-ray diffraction [32], [33] (Fig. 2).
Fig. 2.
The oak ridge thermal ellipsoid plot (ORTEP) figures of 3, 7, 34, 35, 39, and 40, showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 30% probability level, and H atoms are shown as spheres of arbitrary radii.
4. Chemical epimerization and microbial transformations of 20(S)-ginseng saponins
20(R)-ginseng saponins are rare in natural products. They are mainly obtained by chemical epimerization and microbial transformation from their corresponding 20(S)-isomers.
4.1. Chemical epimerization with acid
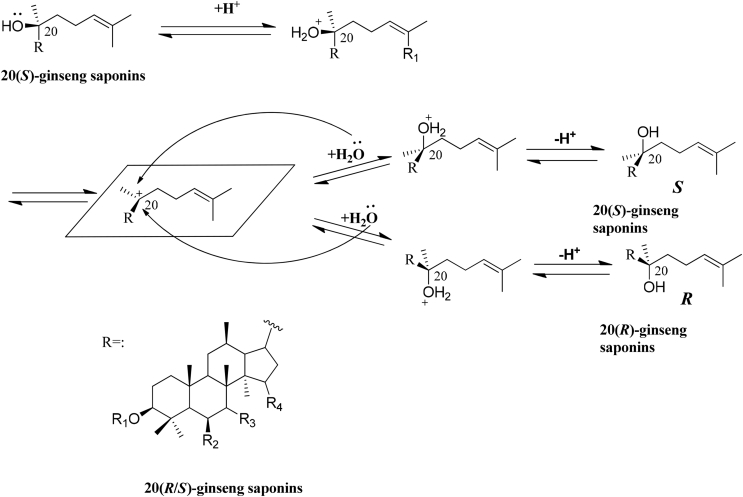
The C20 configuration of ginseng saponins can be epimerized with acid via an SN1 mechanism (Fig. 3). The common acids mainly include dl-tartaric acid, citric acid, lactic acid, acetic acid, etc.
Fig. 3.
Epimerization of 20(S)-ginseng saponins to 20(R)-isomers in acid. The dehydration reaction produced a carbocation intermediate at C-20 of 20(S)-ginseng saponins. Upon rehydration, an oxonium ion was generated, resulting in the conversion of the configuration at C-20 and the creation of 20(R)-ginseng saponins.
Since Compound 2 was extracted from red ginseng in 1980, researchers have found that it is more effective than 20(S)-Rg3 in exhibiting antitumor [8], antioxidative [62], antifatigue [14], neuroprotective [58], and osteoclastogenesis inhibitory effects [16], among others. Therefore, the extraction, synthesis, and evaluation of Compound 2 were prioritized, and the yield was gradually increasing in different optimized conditions. Sun et al [35] obtained Compound 2 at 50% yield by hydrolyzing PPD-type saponins in optimized conditions (dl-tartaric acid, 10 mol/L, 110°C and 2.5 h). Yao et al [36] also acquired Compound 2 from major ginsenosides with a confined microwave-promoted degradation method. The actual Compound 2 yield was predicted to be 94.52% in specific conditions (dl-tartaric acid, 1.19 mol/L, 107.9°C and 2.79 h) through canonical analysis with maximum responses [37].
Compounds 3 and 4 were isolated from the acid hydrolysis product of P. quinquefolium L. for the first time by Ma and Yang [38] and Ma et al [39]. Compound 3 (4.0 g, m.p. 244–245°C) had also been obtained from P. quinquefolium L. (120 g) with 50% citric acid and deglycosylated with sodium hydrate in glycerol in our laboratory. These structures were identified by 1H NMR and 13C NMR [32], [40].
20(R)-Rf (9) was epimerized from ginsenoside Rf by citric acid (pH = 3.5, 90°C, 45 min) and identified by ultra high performance liquid chromatography/time of flight mass spectrometry (UPLC/TOFMS) in red ginseng [30], [34]. The ginsenoside Rf has higher chemical stability than other ginsenosides in acid environments.
Compound 7, 20(R)-dammar-3β,12β,20,25-tetrol (26, m.p. 250–252°C) and 20(R)-dammar-3β,6α,12β,20,25-pentol (27, m.p. 258–260°C) were isolated in the acid hydrolytic products of roots, stems, leaves, fruits, and buds of P. quinquefolium and identified with 13C NMR [41], [42]. Compound 26 was discovered as the derivative of the aglycone of PPD with notable anticancer activity.
20(R)-PD (10, 2.5 mg, m.p. 208–210°C), 20(R)-PT (11,7.5 g, m.p. 267–269°C), 20(R),25-epoxy-3β-acetoxy-12β-hydroxydammarane (12, 80 mg, m.p. 215–217°C), 20(R),25-epoxy-3β,6α-diacetoxy-12β-hydroxydammarane (13, 80 mg, m.p. 285–287°C), 20(R),25-epoxy-3β-acetoxy-6α,12β-dihydroxydammarane (14, 220 mg, m.p. 227–229°C), 20(R),25-epoxy-6α-acetoxy-3β,12β-dihydroxydammarane (15, 50 mg, m.p. 289–291°C), 20(R),25-epoxy-12β-hydroxyammar-3β-O-β-D- glucopyranoside (16, 40 mg, m.p. 227–229°C), 20(R),25-epoxy-3β,6α,12β-trihydroxy dammar-6-O-β-D-glucopyranoside (17, 11 mg, m.p. 212–214°C), 20(R),25-epoxy-3- oxo-6α,12β-dihydroxydammarane (18, 8 mg, m.p. 242–244°C), 20(R)-dammar-3β,12β,20,25-tetrol (26, 20 mg, m.p. 250–252°C), 20(R)-dammar-3β,6α,12β,20,25- pentol (27, 1 g, m.p. 258–260°C), and (20R,24R)-epoxydammar-3β,6α,12β,25-tetraol (35, 40 mg, m.p. 227–229°C) were prepared from P. ginseng stems and leaves (15 kg) with 5% sulfuric acid in alcoholic solution and purified by silica gel column chromatography and SP-HPLC [38]. Their structures were identified by comparison with 1H NMR and 13C NMR data of the corresponding 20(S)-isomers [43], [44], [45], [46], [47].
Zhao et al [48] isolated 20(R)-25-methoxyldammar-3β,12β,20-triol (28), 20(R)-25-methoxyldammar-3β,6α,12β,20-tetrol (29), and 20(R)-20-methoxyldammar- 3β,12β,25-triol (30) from the total hydrolyzed saponins extracted from P. ginseng berries. The structures were elucidated using a combination of 1H NMR, 13C NMR, and MS. Compound 30 showed significant cytotoxic activity against HepG2 (8.78 μM), Colon205 (8.64 μM), and HL-60 (3.98 μM) cell lines. Compound 28 showed a 10-fold to 100-fold greater growth inhibition than 20(R)-Rg3 [anticancer API in Shenyi capsule (YBZ00842003) in China]. Zhang and Zhao [49] found that unsaturated solution and low temperature were beneficial to the separation of 20(R)-25-methoxyl-PPD. They found that under the optimized conditions (acetone, 26 mL, 4°C), the yield and purity of AD-1 crystals were 38.76% and 97.83% from a nonracemic enantiomeric mixture of compound 28 (R: 59.34%; S: 38.35%), respectively.
20(R)-25-acetyldammar-3β,12β,20-triol (31) was first isolated from hydrolysates of stems and leaves of P. notoginseng (Burk) F. H. Chen with hydrochloric acid. It was purified by RP-HPLC (CH3OH/H2O, 88:12) and identified by comparison with the 1H NMR and 13C NMR data of Compounds 25 and 26 [50]. The contents of Compounds 3, 26, and 31 were highest in the stems and leaves of P. notoginseng.
In our previous study, two C-24 epimeric 3-acetylated ocotillol-type saponins, (20R,24S)-epoxy-3-acetyldammar-3β,12β,25-triol (33, m.p. 241–243°C) and (20R,24R)-epoxy-3-acetyldammar-3β,12β,25-triol (34, m.p. 242–244°C), were prepared and isolated from Compound 3 with acetic anhydride in pyridine at room temperature using silica gel column chromatography (ethyl acetate/petroleum ether, 10:1) and crystallized from ethyl acetate. Their structures were confirmed by 1H NMR, 13C NMR, HR-MS, and single-crystal X-ray diffraction [32], [58].
(20R,24R)-epoxydammar-3β,6α,12β,25-tetraol (35, m.p. 289–291°C) and (20R,24S)-epoxydammar-3β,6α,12β,25-tetraol (36, m.p. 225–226°C) were systematically semisynthesized from the residue of Compound 7 by Yang et al [51]. (20R,24R)-epoxydammar-3β,6α,12β,25-tetraol-6-O-β-D-glucopyranoside (37, m.p. 187–189°C) and (20R,24S)-epoxydammar-3β,6α,12β,25-tetraol-6-O-β-D- glucopyranoside (38, m.p. 185–187°C) were separated from the residue of Compound 4. Structures were elucidated based on comprehensive 1H NMR, 13C NMR, heteronuclear singular quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC), rotating frame overhauser enhancement spectroscopy (ROESY), and MS. The configurations at C20 or C24 and the number of glycosyl at C-3 were shown to have an important influence on the compounds' cytotoxicity.
Recently, (20R,24S)-20,24-epoxy-3-oxo-12β,25-dihydroxdammarane (39, m.p. 213–215°C), and (20R,24R)-20,24-epoxy-3-oxo-12β,25-dihydroxydammarane (40, m.p. 223–224°C), (20R,24S)-20,24-epoxy-12-oxo-3β,25-dihydroxydammarane (41, m.p. 251–253°C) and (20R,24R)-20,24-epoxy-12-oxo-3β,25-dihydroxydammarane (42, m.p. 90.5–92.5°C) were synthesized by oxidation and hydrolysis from Compound 3. Their structures were confirmed by hr-ms, 1H NMR, and 13C NMR. Simultaneously, the configurations of Compounds 39 and 40 were authenticated by single-crystal X-ray diffraction [33].
The C20 configuration of ginsenosides is unchanged during alkaline degradation. Consequently, 20(R)-ginseng saponins are primarily obtained by acid epimerization, and some of them possess good pharmacological activities [8] (Table 2). However, some side reactions such as epimerization and hydration were easily induced. The optimization of chemically catalyzed and scaled-up preparation of 20(R)-ginseng saponins still faces tremendous difficulties.
Table 2.
Summary of acid transformation to 20(R)-ginseng saponins
| Entry | Ingredient name | Substrates | Transformation condition | Refs |
|---|---|---|---|---|
| 2 | 20(R)-Rg3 | PPD saponins | D, L-tartaric acid | [35], [36] |
| 3 | 20(R)-PPD | P. ginseng and P. quinquefolium | Sulfuric acid in alcoholic solution (5%) and citric acid (50%) | [32], [38] |
| 4 | 20(R)-Rh1 | P. quinquefolium L. | Sulfuric acid in alcoholic solution (5%) and acetic acid | [38], [39] |
| 7 | 20(R)-PPT | P. ginseng and P. quinquefolium | Sulfuric acid in alcoholic solution (5%) and concentrated hydrochloric acid | [38], [41] |
| 9 | 20(R)-Rf | Ginsenoside Rf | Citric acid (pH 3.5) | [30], [34] |
| 10 | 20(R)-PD | P. ginseng | Sulfuric acid in alcoholic solution (5%) and hydrochloric acid (7%) | [39] |
| 11 | 20(R)-PT | P. ginseng | Sulfuric acid in alcoholic solution (5%) and hydrochloric acid (7%) | [39] |
| 12 | 20(R)25-epoxy-3β-acetoxy-12β-hydroxydammarane | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 13 | 20(R),25-epoxy-3β,6α-diacetoxy-12β-hydroxydammarane | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 14 | 20(R),25-epoxy-3β-acetoxy-6α,12β-dihydroxydammarane | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 15 | 20(R),25-epoxy-6α-acetoxy-3β,12β-dihydroxydammarane | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 16 | 20(R),25-epoxy-12β-hydroxydammar-3β-O-β-D-glucopyranoside | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 17 | 20(R),25-epoxy-3β,6α,12β-trihydroxydammar-6-O-β-D-glucopyranoside | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 18 | 20(R),25-epoxy-3-oxo-6α,12β-dihydroxydammarane | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 26 | 20(R)-dammar-3β,12β,20,25-tetrol | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 27 | 20(R)-dammar-3β,6α,12β,20,25-pentol | P. ginseng | Sulfuric acid in alcoholic solution (5%) | [39] |
| 28 | 20(R)-25-methoxyldammar-3β,12β,20-triol | P. ginseng berry | Hydrochloric acid (18%) | [48] |
| 29 | 20(R)-25-methoxyldammar-3β,6α,12β,20-tetrol | P. ginseng berry | Hydrochloric acid (18%) | [48] |
| 30 | 20(R)-20-methoxyldammar-3β,12β,25-triol | P. ginseng berry | Hydrochloric acid (18%) | [48] |
| 31 | 20(R)-25-acetyldammar-3β,12β,20-triol | P. notoginseng | Concentrated hydrochloric acid | [50] |
| 33 | (20R,24S)-epoxy-3-acetyldammar-3β,12β,25-triol | P. quinquefolium L. | Citric acid (50%) | [32] |
| 34 | (20R,24R)-epoxy-3-acetyldammar-3β,12β,25-triol | P. quinquefolium L | Citric acid (50%) | [32] |
| 35 | (20R,24R)-epoxydammar-3β,6α,12β,25-tetraol | P. ginseng | Alcoholic solution of sulfuric acid (5%) and concentrated sulfuric acid (pH 3–5) | [39], [51] |
| 36 | (20R,24S)-epoxydammar-3β,6α,12β,25-tetraol | P. ginseng | Concentrated sulfuric acid (pH 3–5) | [51] |
| 37 | (20R,24R)-epoxydammar-3β,6α,12β,25-tetraol-6-O-β-D-glucopyranoside | P. ginseng | Concentrated sulfuric acid (pH 3–5) | [51] |
| 38 | (20R,24S)-epoxydammar-3β,6α,12β,25-tetraol-6-O-β-D-glucopyranoside | P. ginseng | Concentrated sulfuric acid (pH 3–5) | [51] |
PPD, protopanaxadiol.
4.2. Microbial transformation
Compounds 3 (328 mg) and 4 (43 mg) could be isolated from fermented ginseng (1 kg) and crystallized by Lactobacillus paracasei A221 containing minor ginsenosides and metabolites of fermentation [52]. Compound 3, the main metabolite of enzymatic reactions of Compound 2 from black ginseng (pH 5.0, 55°C), with 100% conversion, possessed potential antitumor properties [53].
Chen et al [54] produced Compound 4 and 20(R)-R2 (8) from microbial transformation of 20(S)-PPD-type saponins by Absidia coerulea (26°C, 95% ethanol). Compound 8 [m.p. 239–240°C; +17 (c 0.17, MeOH)] was identified as a new metabolite by spectroscopic data [55], [56].
Chen et al [57] investigated the microbial transformation of Compound 10 by Absidia corymbifera AS 3.3387. Seven oxidized and hydroxylated products 20(R),25-epoxy-3-oxo-12β-hydroxydammarane (19), 20(R),25-epoxy-3-oxo- 7β,12β,24α-trihydroxydammarane [20, m.p. 312–313°C, +47.2 (c 0.1, MeOH)], 20(R),25-epoxy-3-oxo-7β,12β,24β-trihydroxydammarane [21, m.p. 334–336°C, +24.7 (c 0.1, MeOH)], 20(R),25-epoxy-3-oxo-12β,15α,24α-trihydroxydammarane [22, m.p. 285–287°C, +18.7 (c 0.1, MeOH)], 20(R),25- epoxy-3-oxo-12β,15α,24β-trihydroxydammarane [23, m.p. 311–313°C, +21.2° (c 0.1, MeOH)], 20(R),25-epoxy-3-oxo-7β,12β,24β-trihydroxyr-15-endammarane [24, m.p. 292–294°C, +26.4 (c 0.1, MeOH)], and 20(R),25-epoxy-3-oxo- 7β,12β,24β-trihydroxy-15,30-cyclodammarane [25, m.p. 352–353°C, +30.3 (c 0.1, MeOH)] were obtained, which are new compounds reported for the first time. Their structures were elucidated by 1H NMR, 13C NMR, HSQC, HMBC, ROESY, and HR-MS. Compounds 23, 24, and 25 exhibited strong cytotoxic activities against four cancer cell lines (Du-145, Hela, HepG2, and MCF-7) and one normal cell line Vero.
Microbial transformation is an impactful approach to diversify natural product structures and prepare a variety of derivatives to search for new lead compounds (Table 3). However, optimizing incubation conditions, microbial transformation conditions, and extraction processes is still a huge challenge.
Table 3.
Summary of microbial transformation of 20(R)-ginseng saponins
| Entry | Ingredient name | Substrates | Transformation condition | Refs |
|---|---|---|---|---|
| 1 | (R)-Rh2 | 20(R)-Rg3 | Aspergillus niger | [53] |
| 3 | 20(R)-PPD | Fermented ginseng, 20(R)-Rg3 | Lactobacillus paracasei A221 and Aspergillus niger | [52], [53] |
| 4 | 20(R)-Rh1 | Fermented ginseng, 20(S)-Rg1 | Lactobacillus paracasei A221 and Absidia coerulea (AS 3.3389) | [52], [54] |
| 8 | 20(R)-R2 | 20(S)-R2 | Absidia coerulea (AS 3.3389) | [54] |
| 19 | 20(R),25-epoxy-3-oxo12β-hydroxydammarane | 20(R)-PD | Absidia corymbifera (AS 3.3387) | [57] |
| 20 | 20(R),25-epoxy-3-oxo7β,12β,24α-trihydroxydammarane | 20(R)-PD | Absidia corymbifera (AS 3.3387) | [57] |
| 21 | 20(R),25-epoxy-3-oxo7β,12β,24β-trihydroxydammarane | 20(R)-PD | Absidia corymbifera (AS 3.3387) | [57] |
| 22 | 20(R),25-epoxy-3-oxo12β,15α,24α-trihydroxydammar | 20(R)-PD | Absidia corymbifera (AS 3.3387) | [57] |
| 23 | 20(R),25-epoxy-3-oxo12β,15α,24β-trihydroxydammarane | 20(R)-PD | Absidia corymbifera (AS 3.3387) | [57] |
| 24 | 20(R),25-epoxy-3-oxo7β,12β,24β-trihydroxy-15-endammarane | 20(R)-PD | Absidia corymbifera (AS 3.3387 | [57] |
| 25 | 20(R),25-epoxy-3-oxo-7β,12β,24β-trihydroxy-15,30-cyclodammarane | 20(R)-PD | Absidia corymbifera (AS 3.3387) | [57] |
PD, panaxadiol.
5. Pharmacological activities of 20(R)-ginseng saponins
5.1. Effect on nervous system
He et al [58] reported that 20(R)-Rg3 (2) could attenuate the neuronal apoptosis caused by cerebral ischemia–reperfusion injury through the downregulation of calpain I and caspase-3. The expression of calpain I and caspase-3 mRNA could significantly be inhibited at doses of 10 and 20 mg/kg.
Li [15] reported that 20(R)-ginseng saponins (10 and 20 μM of Rh1 or Rg2) could suppress 6-hydroxydopamine (6-OHDA) toxicity in SH-SY5Y cells, induce neurite outgrowths in PC-12 cells, and reduce the 6-OHDA–induced phosphorylation of extracellular signal–regulated protein kinase (ERK) (20 μM).
5.2. Protective effects of human umbilical vein endothelial cells (HUVECs) against injury
It has been reported that 20(R)-Rg3 (2) can protect cultured HUVECs from injury by ipopolysaccharide (LPS) and tumor necrosis factor-α (TNF-α). The mechanism might be closely associated with inhibiting the mobilization of cytosolic calcium, attenuating the generation of plasminogenal activator inhibitor-1 (PAI-1), and elevating the tissue-type plasminogen activator (t-PA) level [59], [60]. It could induce HUVEC proliferation at micromolar concentration (10 μM), cell migration, and tube formation in vitro (1-103 μM), as well as ex vivo endothelial sprouting through the activation of protein kinase B/extracellular signal-regulated protein kinase-endothelial nitric oxide synthase (AKT/ERK-eNOS) signaling pathways. Compound 2 (150 and 600 nM) also remarkably reduced basic fibroblast growth factor–induced angiogenesis in an in vivo Matrigel plug assay [61], [62].
Keung et al [11] found that Compound 2 could inhibit angiogenesis. They demonstrated that Rg3-induced angiosuppression (20 nM) could be mediated by miR-520h, which was proposed to negatively regulate angiogenesis through suppressing EphB2 and EphB4 expression.
5.3. Antiinflammatory and antioxidative effects
It had been reported that 20(R)-Rh2 (1; 10, 30, and 50 μM) has matrix metalloproteinase inhibitory, antiinflammatory, and antioxidative activities by inhibiting nitric oxide (NO), prostaglandin E2 (PGE2), reactive oxygen species (ROS) and pro–matrix metalloproteinase-9 (pro-MMP-9) levels [63].
Wei et al [8] found that Compound 2 (10 mg/kg) exhibited significantly higher antioxidant effects than its 20(S)-isomer (10 mg/kg) in mice. It might inhibit Cy-induced oxidative stress in mice and elevate the activities of catalase, superoxidase dismutase, and lysozyme (p < 0.05).
Cheng et al [63] reported that Compound 2 (50 μg/mL) could suppress the early formation of hypertrophic scarring (HS) and later HS hyperplasia by inducing the apoptosis of fibroblasts. Yoon et al [64] found that Compound 2 (20 mM) might act as a dual therapeutic regulator for the treatment of inflammatory and oxidative stress–related diseases and downregulating VEGF expression [26], [66].
20(R/S)-Rg3 exhibited obvious protection against H2O2-induced oxidative stress in SK-N-SH cells, with Compound 2 and 20(S)-Rg3 decreasing ROS formation by 44.1% and 29.2%, respectively. Thus, the 20(R)-isomer displayed better antioxidant activity than 20(S)-isomer in vitro [13].
Fufang Ejiao Syrup (FES, 20(R)-Rg3 present) is an extensively used immune booster in Traditional Chinese Medicine in eastern Asian countries. Compound 2 (50 g/mL) demonstrated cytoprotective effects on bEnd.3 cells against oxidative injury and was discarded to perform the preprotective study [67].
5.4. Antitumor effects
Lv et al [67] found that 20(R)-Rh2 (1) suppressed the growth of H22 transplanted tumors in vivo, and the highest inhibition rate was 46.8% (p < 0.05). Qi et al [68], [69] demonstrated that it could promote A549 cell apoptosis by activating the IκB kinase/nuclear factor-κ-gene binding (I-κB/NF-κB) signaling pathway.
Kim [70] reported that Compound 2 [C57 BL/6 mice, 100 g intravenous (i.v.); 1,000 g per os (p.o.)] could inhibit pulmonary metastasis of B16-BL6 melanoma cells in vitro and in vivo by oral administration.
Compound 2 (100 mM) had a strong inhibitory effect on UGT1A8 and UGT1A1. Compound 2, 20(R)-Rh2, 20(R)-PPD, and 20(R)-Rh1, had no apparent inhibitory effect on UGT1A1 [71]. Kim et al [71] found that Compound 2 (25 and 50 g/mL) could suppress lung cancer migration, invasion, and anoikis resistance of A549 lung cancer cells in vitro by inhibiting the tobuloglomerular feedback-β1 (TGF-β1)–induced epithelial to mesenchymal transition (EMT).
An efficacy study showed that Compound 2 (3 mg/kg) could significantly inhibit the growth of H22 transplanted tumors in mice, and the inhibition rate of tumor growth was 40.9% [72], [73].
Compound 2 notably improved the clinical therapeutic efficacy and quality of life of patients. The clinical relief rate of patients treated with antiangiogenic agent Compound 2 (36.6%) was higher than that of the patients not treated with it (16.7%, p < 0.05). It provided better therapeutic efficacy against early-stage cancer than in advanced stages (p < 0.05) [60].
Compounds 35, 36, 37, and 38 (ocotillol-type saponins) were synthesized from 20(R)-Rh1 (4) and 20(R)-PPT (7). They possessed better cytotoxicity (200 μg/mL) against HeLa, A549, and MCF-7 cells (>90%) than their corresponding 20(S)-isomer, suggesting that the carbonyl group at C3 might improve their cytotoxicity [74].
Shenyi capsule (20(R)-Rg3, API) inhibited the proliferation of HO-8910PM cells and the apoptosis of melanoma B16-4A5 cells [75]. Huang et al [75] found that it could treat advanced primary liver cancer, with treatment group survival and efficiency rates of 90% and 70%, respectively.
5.5. Other effects
20(R)-Rh2 (1) might play a large role in selective osteoclastogenesis inhibition. Liu et al [16] reported that Compound 1 showed selective osteoclastogenesis inhibitory activity without any cytotoxicity (RAW264 cells) up to 100 μM in vitro. However, the mechanism was ambiguous and needs further research.
Tang et al [14] predicted a benefit of Compound 2 (0.1 and 0.5 mg/kg) as an antifatigue treatment by intranasal administration in mice (p < 0.05). The mechanism was related to the increase of the storage of hepatic glycogen and the decrease of the accumulation of metabolites such as lactic acid and serum urea nitrogen.
Compared with other ginsenosides, 20(R)-ginseng saponins possessed better pharmacological activities, such as antioxidant, cytotoxicity, and osteoclastogenesis inhibitory effects. Some studies found that Compounds 1 and 2 have better osteoclastogenesis inhibitory and antioxidative activities than the corresponding 20(S)-isomers. Shenyi capsule (API: 20(R)-Rg3) and FES (API: 20(R)-Rg3) were approved by the China Food and Drug Administration (CFDA) in China as antitumor and immune-boosting drugs. However, the mechanisms of actions are not clear and require investigation.
6. Metabolism of 20(R)-ginseng saponins
The absorption of Compound 2 (3.2 mg/kg) was rapid in the human body, and its elimination was rapid after oral administration in vivo. The pharmacokinetic results showed that it exhibited first-order kinetic characteristics in 14 healthy volunteers [76], [77].
Mami [55] found that Compound 2 (100–1000 μg/mouse) could induce a significant decrease in lung metastasis of B16-BL6 melanoma by oral administration. The mechanism of the antimetastatic effect was related to inhibition of the lung and invasion of tumor cells and to antiangiogenesis activity.
Anaerobic incubation of Compound 2 quickly yielded 20(R)-Rh2 (1) or 20(R)-PPD (3) with bacteria isolated from human fecal microflora, Bacteroides sp., Eubacterium sp., and Bifidobacterium sp. However, Fusobacterium sp. could metabolize Rg3 only to Rh2. Compound 3 (50–100 mg/mL) potently inhibited the growth of Helicobacter pylori [78].
Peng et al [78] found that 20(R/S)-Rg3 could be deglycosylated to their corresponding chiral metabolites, Compound 1 or Compound 3, to different extents. However, Compound 3 can undergo single-direction chiral inversion to 20(S)-Rg3 in rats. The chiral inversion rate was calculated to be 7.9% after i.v. administration and was estimated to be greater than 9.7% after i.g. administration. Stereo-selective pharmacokinetic parameters, metabolic degrees, and chiral inversion extents of Rg3-epimers in rats were also discussed for the first time.
20(R)-Rh1 was identified as an Re metabolite in rats. The microbial transformation metabolites of Re were used as standard references, and they were identified by HPLC-ESI-MS/MS [79].
Pharmacokinetic study showed that 20(R)-Rg2 (2 mg/kg) was rapidly absorbed and eliminated in plasma after i.v. administration by a simple and reproducible HPLC method [80].
Many studies reported that the absorption, distribution, metabolism, and excretion of 20(R)-ginseng saponins are rapid in vivo. 20(S)-ginseng saponins can be transformed to 20(R)-isomers by various microorganisms in vivo. However, the microbial transformation condition is difficult in vitro and needs further exploration.
7. Conclusion and perspectives
Panax ginseng, known as Koran ginseng, one of the most commonly used traditional plants, has shown a wide range of pharmacological applications. Ginseng saponins are the major active ingredients found in ginseng and are responsible for biological and pharmacological activities, such as antioxidation, antiinflammation, vasorelaxation, and anticancer actions. Our understanding of 20(R)-ginseng saponins has advanced tremendously in the last few years. Investigations related to pharmacological and stereo-selective activities of 20(R)-ginseng saponins have been helpful in verifying the functions and values of the corresponding 20(S)-isomers, such as antitumor, antioxidant, cytotoxicity and osteoclastogenesis inhibitory effects. The effects of 20(R)-ginseng saponins in cancer chemoprevention and therapy are one of the most popularly studied areas, and the curative effects are known in clinical terms. Shenyi capsule (YBZ00842003, 20(R)-Rg3 as API) and FES (Z37021371, API: 20(R)-Rg3), approved by CFDA, combined with some chemotherapeutics, have remained the favorable treatment for primary lung cancer and liver cancer and improving immunity. Based on our review, 20(R)-ginseng saponins function as anticancer agents because of their antiproliferative, antiinflammatory, and antioxidant effects. They have not only great potential capacity for varied mechanisms for cancer treatment but also a lack of toxicity to normal cells, making 20(R)-ginseng saponins appealing candidates for drug development.
To date, studies have mostly focused on identifying and purifying single 20(R)-ginseng saponins and investigating their pharmacological activities and molecular mechanisms in cell lines and animal models. Few 20(R)-ginseng saponins have been tested in humans despite the fact that they are widely accepted to have therapeutic effects when used alone or in combination with other therapeutic agents in the management of a wide range of chronic diseases. Therefore, studies demonstrating the therapeutic effects of ginseng and 20(R)-ginseng saponins are in high demand. Moreover, further studies using single 20(R)-ginseng saponins need to include the molecular and cellular mechanisms of action, specificity, structure–function relationship, pharmacokinetic profile, and toxicity in animal models and humans. These studies could maximize the potential of 20(R)-ginseng saponins as promising herbal medicines, thereby further contributing to the promotion of global health. There are still many challenges in the optimization of chemical semisynthesis, microbial transformation, and scaled separation of optical 20(R)-ginseng saponins. A number of recent studies have presented evidence showing that fermentation by microorganisms is the most effective and environmental transformation mode for the separation of 20(R)-ginseng saponins. Some metabolites produced by the action of microbes have different structures from those of naturally occurring 20(R)-ginseng saponins in vivo or vitro. Microbial transformation may well become a research hot spot, opening a new avenue for ginseng development in the future. Therefore, further studies are needed to optimize microbial transformation conditions and scale up separation techniques of 20(R)-ginseng saponins.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 81473104) and Shandong Graduate Education Innovation Program of China (SDYY16132).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.01.005.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Park J., Song H., Kim S.K., Lee M.S., Rhee D.K., Lee Y.J. Effects of ginseng on two main sex steroid hormone receptors: estrogen and androgen receptors. J Ginseng Res. 2017;41:215–221. doi: 10.1016/j.jgr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garriques S.S. On panaquilon, a new vegetable substance. Ann Chem Pharm. 1854;90:231–234. [Google Scholar]
- 4.Shibata S., Tanaka O., NagaI M., Ishit T. Studies on the constituents of Japanese and Chinese crude drugs. XII. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull (Tokyo) 1963;11:762–765. doi: 10.1248/cpb.11.762. [DOI] [PubMed] [Google Scholar]
- 5.Gui Y., Ryu G.H. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder) J Ginseng Res. 2014;38:146–153. doi: 10.1016/j.jgr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.M., Bae B.S., Park H.W. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y.M., Yoon H., Park H.M. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J Ginseng Res. 2017;41:113–119. doi: 10.1016/j.jgr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei X., Su F., Su X., Hu T.J., Hu S.H. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83:636–642. doi: 10.1016/j.fitote.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. Chemical studies on crude drug precession.I. on the constituents of ginseng radix rubra (1) Yakugaku Zasshi. 1983;103:612–622. [PubMed] [Google Scholar]
- 10.Chen Y.J., Xu R.X., Ma Q.F., Pei Y.P., Xie H., Yao X.S. Studies on new minor saponins isolated from leaves of panax ginseng C.A. Meyer. Acta Pharmaeeutiea Siniea. 1987;22:655–689. [PubMed] [Google Scholar]
- 11.Keung M.H., Chan S., Kwok H., Ricky H., Wong N.S., Yue Y.K. Role of microRNA-520h in 20(R)-ginsenoside-Rg3-mediated angiosuppression. J Ginseng Res. 2016;40:151–159. doi: 10.1016/j.jgr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H., Liu H., Bai J. Simultaneous determination of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re and 20(S) protopanaxatriol in beagle dog plasma by ultra high performance liquid mass spectrometry after oral administration of a Panax notoginseng saponin preparation. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;974:42–47. doi: 10.1016/j.jchromb.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Li G., Zhang X.X., Lin L., Liu X.N., Ma C.J., Li J., Wang C.B. Preparation of ginsenoside Rg3 and protection against H2O2-induced oxidative stress in human neuroblastoma SK-N-SH cells. J Chem. 2014;2014:1–6. [Google Scholar]
- 14.Tang W.Y., Zhang Y., Gao J., Ding X.Y., Gao S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol Pharm Bull. 2008;31:2024–2027. doi: 10.1248/bpb.31.2024. [DOI] [PubMed] [Google Scholar]
- 15.Li X.F., Lui C., Jiang Z.H., Ken Y. Neuroprotective effects of ginsenosides Rh1 and Rg2 on neuronal cells. Chin Medi. 2011;6:19. doi: 10.1186/1749-8546-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Shiono J., Shimizu K., Yu H.S., Zhang C.Z., Jin F.X., Kondo R. 20(R)-ginsenoside Rh2, not 20(S), is a selective osteoclastgenesis inhibitor without any cytotoxicity. Bioorg Med Chem Lett. 2009;19:3320–3323. doi: 10.1016/j.bmcl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., Xu Y.R., Yang J.J., Wang W.Z., Zhang J.Q., Zhang R.M., Meng Q.G. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J Ginseng Res. 2017;41:373–378. doi: 10.1016/j.jgr.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao P.Y., Wang D., Zhang Y.J. Dammarane-type glycosides from steamed notoginseng. J Agric Food Chem. 2008;56:1751–1756. doi: 10.1021/jf073000s. [DOI] [PubMed] [Google Scholar]
- 19.Dou D.Q., Li W., Guo N., Fu R., Pei Y.P., Koike K.Z., Nikaido T. Ginsenoside Rg8, a new dammarane-type triterpenoid saponin from roots of panax quinquefolium. Chem Pharm Bull. 2006;54:751–753. doi: 10.1248/cpb.54.751. [DOI] [PubMed] [Google Scholar]
- 20.Xiang W.J., Guo C.Y., Ma L., Hu L.H. Dammarane-type glycosides and long chain sesquiterpene glycosides from Gynostemma yixingense. Fitoterapia. 2010;81:248–252. doi: 10.1016/j.fitote.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Yang X.W., Li K.K., Zhou Q.L. 20(S)-ginsenoside-Rf2, a novel triterpenoid saponin from stems and leaves of manax ginseng. Chin Tradit Herbal Drugs. 2015;46:3137–3145. [Google Scholar]
- 22.In G., Ahn N.-G., Bae B.-S. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J Ginseng Res. 2017;41:361–369. doi: 10.1016/j.jgr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung W.K., Sang B.H., Ho P. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J Chromatogr A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang D., Liao P.Y., Zhu H.T., Chen K.K., Xu M., Zhang Y.J., Yang C.R. The processing of Panax notoginseng and the transformation of its saponin components. Food Chem. 2012;132:1808–1813. [Google Scholar]
- 25.Xu S.X., Wang N.L., Li Y.H. Studies on the chemical constituents of chinesered ginseng (Ⅱ) Acta Pharmaceutical Sinica. 1986;21:356–360. [PubMed] [Google Scholar]
- 26.Park J.D., Lee Y.H., Kim S. Ginsenoside Rf2, a new dammarane glycoside from Korean red ginseng (Panax ginseng) Arch Pharm Res. 1998;21:615–617. doi: 10.1007/BF02975384. [DOI] [PubMed] [Google Scholar]
- 27.Katritzky A.R., Akhmedov N.G., Wang M. Spectral assignments and reference data. Springer; US: 2002. pp. 478–480. [Google Scholar]
- 28.Liu J.P., Lu D., Li P.Y. A novel hexanordammarane glycoside from the leaves and stems of Panax quinquefolium L. Nat Prod Res. 2012;26:744–748. doi: 10.1080/14786419.2010.551768. [DOI] [PubMed] [Google Scholar]
- 29.Shen R., Cao X., Laval S., Sun J.S., Yu B. Synthesis of ocotillol-type ginsenosides. J Org Chem. 2016;81:10279–10294. doi: 10.1021/acs.joc.6b01265. [DOI] [PubMed] [Google Scholar]
- 30.Asakawa J., Kasai R., Yamasaki K., Tanaka O. 13C NMR Study of ginseng sapogenins and their related dammarane type triterpenes. Tetrahedron. 1977;33:1935–1939. [Google Scholar]
- 31.Yang H.J., Kim J.Y., Kim S.O., Young H.Y., Sang H.S. Complete 1H-NMR and 13C-NMR spectral analysis of the pairs of 20(S) and 20(R) ginsenosides. J Ginseng Res. 2014;38:194–202. doi: 10.1016/j.jgr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J.J., Xu Y.R., Li X.L., Zhang K.X., Zhang R.M., Wang W.Z., He X.Y., Meng Q.G., Hou G.G. Synthesis and crystal structures of two C24 epimeric 3-acetyled 20(R)-ocotillol type sapogenins obtained from 20(R)-protopanaxadiol. J Chem Res. 2016;40:235–238. [Google Scholar]
- 33.Liu Z., Xu Y.R., An X.S., Yang J.J., Meng Q.G., Hou G.G. Synthesis and crystal structure of ocotillol-type metabolites derived from (20R)-protopanaxadiol. J Chem Res. 2017;41:216–220. [Google Scholar]
- 34.Lee S.M. The mechanism of acid-catalyzed conversion of ginsenoside Rf and two new 25-hydroxylated ginsenosides. Phytochem Lett. 2014;10:209–214. [Google Scholar]
- 35.Sun C.P., Gao W.P., Zhao B.Z., Cheng L.Q. Catalytic conversion of protopanaxadiol saponins to 20(R)-ginsenoside Rg3 by tartaric acid. Chin Tradit Herbal Drugs. 2013;44:1893–1898. [Google Scholar]
- 36.Yao H., Jin Y., Yang J. Conversion rule of rare ginsenosides produced from major ginsenosides by confined microiwave promoted. Chem J Chin Univ. 2014;35:2317–2323. [Google Scholar]
- 37.Sun C., Gao W., Zhao B., Cheng L. Optimization of the selective preparation of 20(R)-ginsenoside Rg3 catalyzed by d,l-tartaric acid using response surface methodology. Fitoterapia. 2013;84:213–221. doi: 10.1016/j.fitote.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Ma L.Y., Yang X.W. Chemical constituents in acid hydrolysates of total saponins from stems and leaves of Panax ginseng. Chin Traditl Herbal Drugs. 2015;46:2522–2533. [Google Scholar]
- 39.Ma X.N., Chai R.H., Zhao Y.Q. Rare constituents with anti-cancer activity extracted from hydrolytic products in saponins. Chin Traditl Herbal Drugs. 2008;39:1291–1294. [Google Scholar]
- 40.Xu Y.R., Yang J.J., Liu J., Houb G.G., Meng Q.G. Synthesis and crystal structures of C24-epimeric 20(R)-ocotillol-type saponins. Acta Crystallogr C Struct Chem. 2016;72:498–503. doi: 10.1107/S2053229616007270. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y.G., Lv Y.P., Gui S.H., Wen J., Li G.X. Preparation of protopanaxadiol and its epimer 20(R)-protopanaxadiol from leaf saponins of Panax Notoginseng. Fine Chemicals. 2003;20:425–426. [Google Scholar]
- 42.Zhao Y.Q., Yuan C.L., Fu Y.Q. Chemical studies of minor triterpene compounds isolated from the stems and leaves of panax Ginseng C. A. Meyer. Acta Pharmaceutica Sinica. 1990;25:299–301. [PubMed] [Google Scholar]
- 43.Atopkina L.N., Denisenko V.A. Synthesis of panaxatriol glucosides. Chem Nat Compd. 2009;45:664–672. [Google Scholar]
- 44.Atopkina L.N., Denisenko V.A. Glycosylation of panaxadiol. Chem Nat Compd. 2011;46:892–896. [Google Scholar]
- 45.Nguyen M.D., Nguyen T.N., Ryoji K. Saponins from vietnamese ginseng, panax vietnamensis collected in central vietnam. Chem Pharm Bull. 1993;41:2010–2014. doi: 10.1248/cpb.41.2010. [DOI] [PubMed] [Google Scholar]
- 46.Jia J.M., Wang Z.Q. Studies on the chemical constituents of the saponins from Panax quinquefolium. Chin Tradit Herbal Drugs. 2009;40:1204–1207. [Google Scholar]
- 47.Shi S.M., Li W., Cao J.Q. Studies on the chemical constituents of Pseudo-ginseng. Chin Tradit Herbal Drugs. 2010;41:1249–1251. [Google Scholar]
- 48.Zhao J.M., Li N., Zhang H., Wu C.F., Piao H.R., Zhao Y.Q. Novel dammarane-type sapogenins from Panax ginseng berry and their biological activities. Bioorg Med Chem Lett. 2011;21:1027–1031. doi: 10.1016/j.bmcl.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S.N., Zhao Y.Q. Crystallization separation of antitumor constituent 20(R)-25-OCH3-PPD. Chin Tradit Herbal Drugs. 2014;45:770–773. [Google Scholar]
- 50.Zhang H.M., Li S.L., Zhang H. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Yang J., Li X., Sun T., Gao Y., Chen Y.X., Jin Y.R., Li Y. Semisynthesis and bioactive evaluation of oxidized products from 20(S)-ginsenoside Rg3, Rh2, protopanaxadiol (PPD) and their 20(R)-epimers as cytotoxic agents. Steroids. 2016;106:26–34. doi: 10.1016/j.steroids.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Igami K., Shimojo Y., Ito H. Fermented ginseng contains an agonist of peroxisome proliferator activated receptors α and γ. J Med Food. 2016;19:817–822. doi: 10.1089/jmf.2016.3673. [DOI] [PubMed] [Google Scholar]
- 53.Liu L., Zhu X.M., Wang Q.J. Enzymatic preparation of 20(S,R)-protopanaxadiol by transformation of 20(S,R)-Rg3 from black ginseng. Phytochemistry. 2010;71:1514–1520. doi: 10.1016/j.phytochem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Chen G.T., Yang M., Lu Z.Q., Zhang J.Q., Huang H.L., Liang Y., Guan S.H., Song Y. Microbial transformation of 20(S)-protopanaxatriol-type saponins by Absidia coerulea. J Nat Prod. 2007;70:1203–1206. doi: 10.1021/np070053v. [DOI] [PubMed] [Google Scholar]
- 55.Mami M., Yung C.Y., Kaori M., Katsuaki S., Ikuo S., Shuichi T., Keiichi S., Ichiro A. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)-and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 56.Wei J.X., Chang L.Y., Wang J.F., Edmund F., Monika J., Heinrich P., Chen W.A., Eberhard B. Zwei neue dammaran-sapogenine aus den blättern von Panax notoginseng. J Med Plants Res. 1982;45:167–171. doi: 10.1055/s-2007-971367. [DOI] [PubMed] [Google Scholar]
- 57.Chen G., Ge H., Li J. Microbial transformation of 20(R)-panaxadiol by Absidia corymbifera AS 3.3387. J Mole Catalysis B: Enzymatic. 2016;123:154–159. [Google Scholar]
- 58.He B., Chen P., Yang J.Y., Yun Y., Zhang X.C., Yang R.H., Shen Z.Q. Neuroprotective effect of 20(R)-ginsenoside Rg3 against transient focal cerebral ischemia in rats. Neurosci Lett. 2012;526:106–111. doi: 10.1016/j.neulet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Yu Z., Chen P., Yang G.M., Yang L., Shen Z.Q. Protective effects of 20(R)-ginsenoside Rg3 on human umbilical vein endothelial cell injury induced by tumor necrosis factor-α. South Chin J Cardiovasc Dis. 2011;17:198–212. [Google Scholar]
- 60.Kwok H.H., Guo G.L., Lau J.K. Stereoisomers ginsenosides-20(S)-Rg(3) and -20(R)-Rg(3) differentially induce angiogenesis through peroxisome proliferator-activated receptor-gamma. Biochem Pharmacol. 2012;83:893–902. doi: 10.1016/j.bcp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 61.Yue P.Y., Wong D.Y., Wu P.K., Leung P.Y., Mak N.K., Yeung H.W. The angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 62.Choi W.Y., Lim H.W., Lim C.J. Anti-inflammatory, antioxidative and matrix metalloproteinase inhibitory properties of 20(R)-ginsenoside Rh2 in cultured macrophages and keratinocytes. J Pharm Pharmacol. 2013;65:310–316. doi: 10.1111/j.2042-7158.2012.01598.x. [DOI] [PubMed] [Google Scholar]
- 63.Cheng L., Sun X., Hu C., Rong J., Sun B.S., Shi Y.M., Zhang L., Cui W.G., Zhang Y.G. In vivo inhibition of hypertrophic scars by implantable ginsenoside-Rg3-loaded electrospun fibrous membranes. Acta Biomater. 2013;9:9461–9473. doi: 10.1016/j.actbio.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 64.Yoon S.J., Park J.Y., Choi S., Lee J.B., Jung H.Y., Kim T.D., Yoon S.R., Choi I., Shim S.B., Park Y.G. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem Biophys Res Commun. 2015;463:1184–1189. doi: 10.1016/j.bbrc.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 66.Shen L.J., Chen H.L., Zhu Q.F., Wang Y.Y., Wang S.S., Qian J., Wang Y., Qu H.B. Identification of bioactive ingredients with immuno-enhancement and anti-oxidative effects from Fufang-Ejiao-Syrup by LC–MSn combined with bioassays. J Pharmaceut Biomed. 2016;6:363–371. doi: 10.1016/j.jpba.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Lv Q., Rong N., Liu L.J., Xu X.L., Liu J.T., Jin F.X., Wang C.M. Antitumoral activity of (20R)- and (20S)-ginsenoside Rh2 on transplanted hepatocellular carcinoma in mice. Planta Med. 2016;82:705–711. doi: 10.1055/s-0042-101764. [DOI] [PubMed] [Google Scholar]
- 68.Qi X.D., Hou J.C., Yu H.T., Zhang C.J. 20(S)-ginsenoside-Rh2 and 20(R)-ginsenoside-Rh2 activate IκB phosphorylation expression in human lung adenocarcinoma A549 cells. Adv Mate Res. 2011;268–270:1205–1210. [Google Scholar]
- 69.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16:S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D., Zheng Y.F., Min J.S., Parka J.B., Bae S.H., Yoon K.D., Chin Y.W., Oh E., Bae S.K. In vitro stereoselective inhibition of ginsenosides toward UDP-glucuronosyltransferase (UGT) isoforms. Toxicol Lett. 2016;259:1–10. doi: 10.1016/j.toxlet.2016.07.108. [DOI] [PubMed] [Google Scholar]
- 71.Kim Y.J., Choi W.I., Jeon B.N., Choi K.C., Kim K., Kim T.J., Ham J., Jang H.J., Kang K.S., Ko H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014;322:23–33. doi: 10.1016/j.tox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Wu R.H., Ru Q., Chen L., Ma B.M., Li C.Y. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J Food Sci. 2014;79:1430–1435. doi: 10.1111/1750-3841.12518. [DOI] [PubMed] [Google Scholar]
- 73.Yang J., Yu X., Cai X., Chen Y.X., Zang H.M., Li X.W., Jin Y.G. Semisynthesis and cytotoxicity evaluation of a series of ocotillol type saponins and aglycones from 20(S)-ginsenoside Rg2, Rh1, protopanaxatriol and their 20(R)-epimers. Chem Res Chin Univ. 2016;32:35–40. [Google Scholar]
- 74.Sun B.S., Zhang M., Liu L.L. The effects of Rg3 on the apoptosis of melanoma B16-4A5 cells. Chin J Lab Diagn. 2010;14:526–527. [Google Scholar]
- 75.Huang J.Y., Fan Q.X., Sun Y. Recent clinical observation of Cidan Capsule with ginsenoside Rg3 in advanced primary liver cancer. Chin Tradit Patent Med. 2009;31:673–676. [Google Scholar]
- 76.Pang H., Wang H.L., Fu L., Su C.G. Pharmacokinetics of 20(R)-ginsenoside Rg3 in human volunteers. J Chin Pharm Sci. 2001;10:140–143. [PubMed] [Google Scholar]
- 77.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 78.Peng M., Li X., Zhang T., Ding Y., Yi Y.X., Le J., Yang Y.J., Chen X.J. Stereoselective pharmacokinetic and metabolism studies of 20(S)- and 20(R)-ginsenoside Rg3 epimers in rat plasma by liquid chromatography-electrospray ionization mass spectrometry. J Pharm Biomed Anal. 2016;121:215–224. doi: 10.1016/j.jpba.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 79.Chen G.T., Yang M., Guo D.A. Metabolics tudy of ginsenoside Re in rat. Chin J Chin Mat Med. 2009;34:1540–1543. [PubMed] [Google Scholar]
- 80.Gui F.J., Yang X.W., Li L.Y., Tian J.M. Simultaneous enantiomer determination of 20(R)- and 20(S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:1–6. doi: 10.1016/j.jchromb.2006.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.