Figure 4.
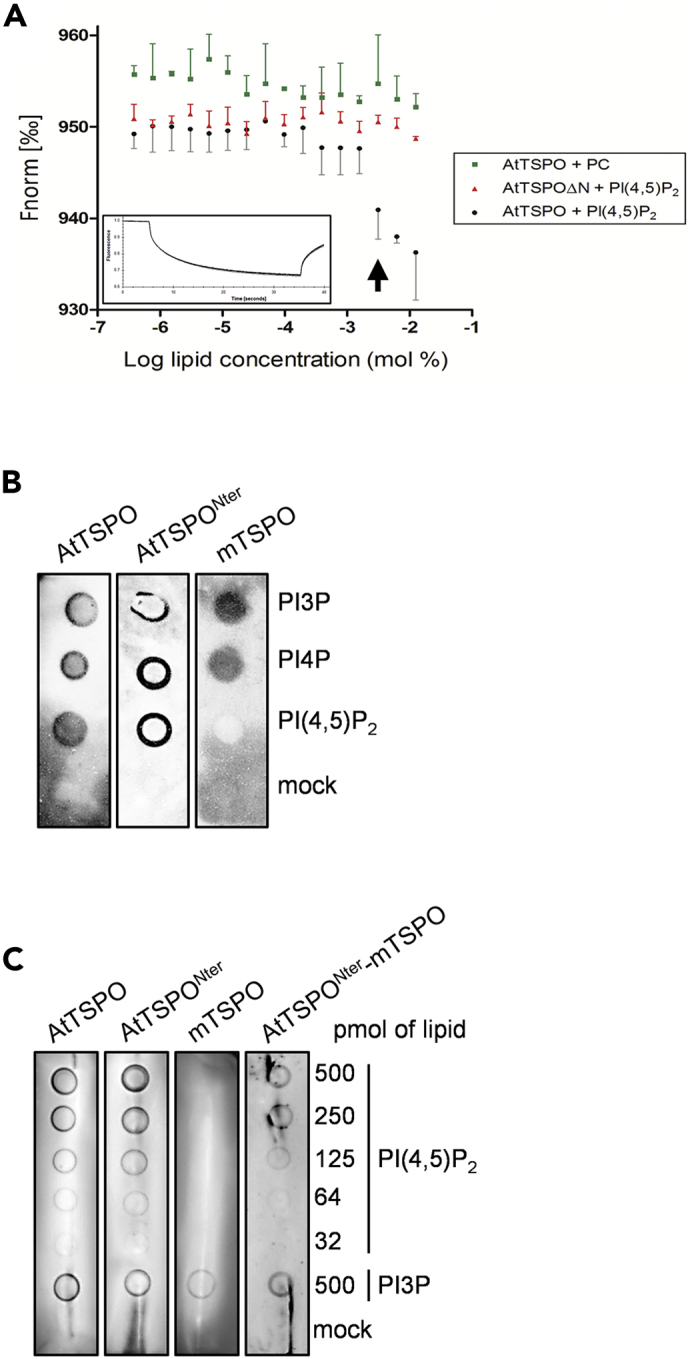
The AtTSPO N-terminal Peptide Binds PI(4,5)P2
(A) N-terminally truncated AtTSPO cannot bind PI(4,5)P2in vitro. Thermophoresis was performed by titrating full-length and N-terminally deleted TSPO against different PI(4,5)P2 concentrations. Protein samples were labeled at polyhistidine tags by RED-tris-NTA. Thermophoretic movement of labeled TSPO increases (normalized fluorescence decreases) upon binding PI(4,5)P2. Phosphatidylcholine served as a negative control lipid. Both datasets (AtTSPO + PI(4,5)P2 and AtTSPOΔN + PI(4,5)P2) were compared using two-way ANOVA followed by Bonferroni multiple comparison post-hoc tests. The threshold lipid concentration yielding statistically significant data (p <0.01) is 1.56 mM (arrow). The non-linear regression-based estimate of the Kd for the AtTSPO-PI(4,5)P2 interaction is 2.3 ± 0.7 mM. The rectangular insert is a representative fluorescence trace. For each sample shown is the mean ± SD of three experiments.
(B) Purified mouse TSPO homolog lacking the plant-specific N-terminus does not bind PI(4,5)P2in vitro, unlike the full-length plant protein and isolated N-terminal peptide. Five hundred pmol of phosphoinositide was spotted on a nitrocellulose membrane, incubated with purified proteins and detected with anti-AtTSPO (for isolated N-terminus) or HisProbe (for AtTSPO and mTSPO). A mock control of 2% n-dodecyl-β-D-maltoside (DDM) was included.
(C) Chimeric mouse TSPO fused to the AtTSPO N-terminus binds PI(4,5)P2in vitro. Purified proteins were incubated with different PI(4,5)P2 concentrations and detected using anti-AtTSPO antibodies (for isolated N-terminal peptide and N-terminus-fused mTSPO) and HisProbe (for AtTSPO and mTSPO). PI3P was spotted as a positive control for mTSPO binding. DDM (2%) served as a mock control. Experiments in (B) and (C) were performed at least twice.
