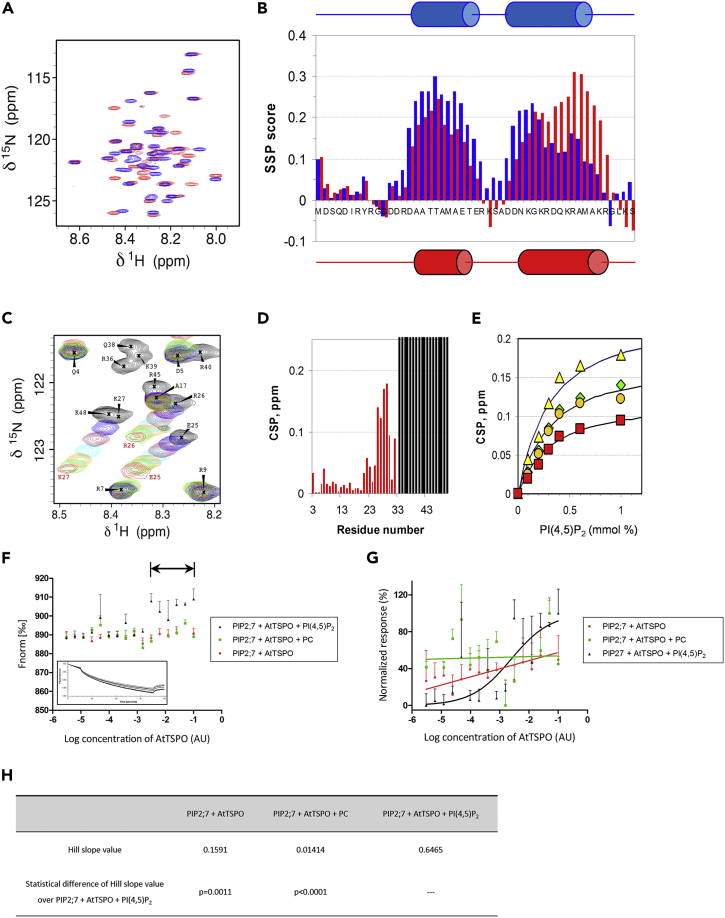
Figure 6.
The Recombinant AtTSPO N-terminal Peptide Interacts with PI(4,5)P2 and This Anionic Lipid Is Required for AtTSPO Interaction with the Aquaporin PIP2;7 In Vitro
(A) 2D 1H-15N HSQC spectrum (500 MHz, 30°C) of 100 μM AtTSPONter in the absence (blue) and in the presence (red) of 25 mM DMPG/50 mM DHPC bicelles.
(B) Secondary Structure Propensity (SSP) score in the absence (blue) and in the presence (red) of DMPG/DHPC bicelles.
(C) Selected region in 2D 1H-15N HSQC showing different behaviors of residues upon titration of AtTSPONter with increasing amounts of PI(4,5)P2 (0 mM, black; 0.1 mM, blue; 0.2 mM, maroon; 0.3 mM, cyan; 0.4 mM, orange; 0.6 mM, green; 1 mM, red). Residues R36, Q38, K39, R40, and K48 disappear at the first addition of PI(4,5)P2, whereas residues E25, R26, and K27 show progressive chemical shift perturbations.
(D) 1H,15N Chemical Shift Perturbations (CSP) after addition of 1 mM PI(4,5)P2. Residues 34–49 that disappear at the first point of the titration are indicated by gray shading. CSPs were calculated as |Δδ(1H)| + |Δδ(15N)|/10.
(E) CSP of E26 (green diamonds), R27 (orange circles), K28 (yellow triangles), and A30 (red squares) as a function of PI(4,5)P2 concentration added.
(F) Thermophoresis analyses of YFP-PIP2;7 titrated against different AtTSPO concentrations. PIP2;7 movement was followed by YFP fluorescence. Either 50 μM PI(4,5)P2, phosphatidylcholine (PC, negative control), or no lipid was added. Thermophoretic motion of Venus-PIP2;7 decreases (normalized fluorescence increases) upon binding of AtTSPO in the presence of 50 μM PI(4,5)P2 but not PC, nor in the absence of lipids. The horizontal arrow indicates the bound state plateau region. The rectangular insert is a representative fluorescence trace. For each sample shown is the mean ± SD of three experiments.
(G) Data from (F) were normalized and fitted using non-linear regression, yielding a typical sigmoidal binding curve only in the presence of PI(4,5)P2.
(H) Statistical comparison of Hill slope values from (G). Low pvalues indicate no similarity in steepness between fitted curves.