Fig. 5.
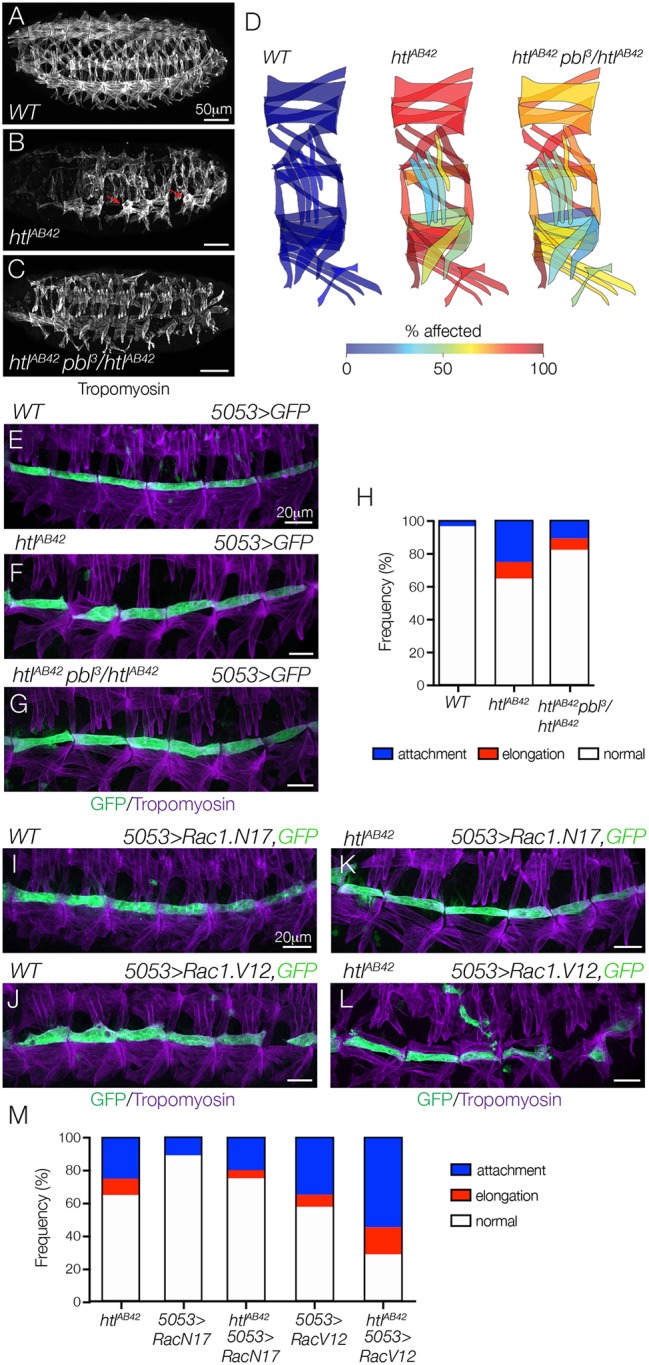
Htl regulates myotube guidance through the Pbl-Rac signaling pathway. (A-C) Stage 16 embryos labeled for Tropomyosin. Body wall muscle defects in htlAB42 embryos (B, red arrows) were suppressed in htlAB42 embryos heterozygous for pbl3 (C), compared with WT (A). (D) Heat map showing the frequency of muscle defects (n=60 segments per genotype) in WT (left) and htlAB42 (middle) embryos, and htlAB42 embryos heterozygous for pbl3 (right). (E-G) Stage 16 5053>eGFP embryos labeled for GFP (green) and Tropomyosin (violet). htlAB42 VL1 myotube guidance defects (F) were suppressed in htlAB42 embryos heterozygous for pbl3 (G), compared with WT (E). (H) Histogram of muscle phenotypes in stage 16 embryos (n≥54 muscles per genotype). (I-L) Stage 16 5053>eGFP embryos labeled for GFP (green) and Tropomyosin (violet). VL1 myotubes that expressed DN Rac1 (Rac1.N17, I) showed normal morphology; VL1 myotubes that expressed CA Rac1 (Rac1.V12, J) showed guidance defects. htlAB42 myotube guidance defects were suppressed in VL1 muscles that expressed Rac1.N17 (K); htlAB42 myotube guidance defects were dramatically enhanced in VL1 muscles that expressed Rac1.V12 (L). (M) Histogram of muscle phenotypes in stage 16 embryos (n≥54 muscles per genotype). See also Fig. S4. Embryos are oriented with anterior to the left and dorsal to the top.
