Fig. 4. Comparison of the structures of M1R-G11 and M2R-GoA.
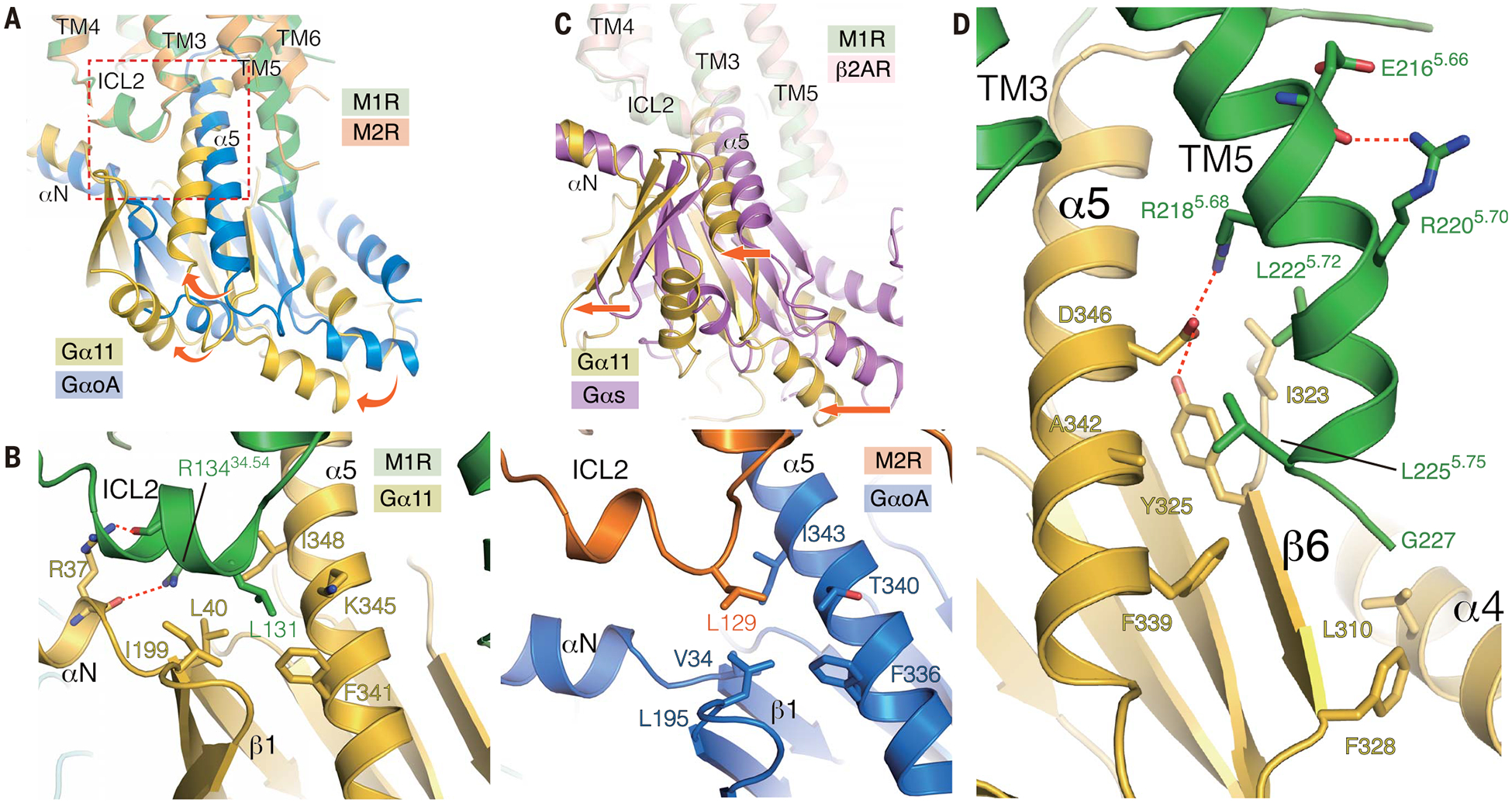
(A) Superposition of M1R-G11 and M2R-GoA complexes with the alignment based on the receptor. Rotational shift from GoA to G11 is depicted with curved arrows. Enlarged area in the panel (B) is shown as a broken rectangle. (B) View of ICL2 interface between the G protein on M1R-G11 (left) and M2R-GoA (right). (C) Superposition of β2AR-Gs and M1R-G11 complexes with the alignment based on the receptor. A translational shift is shown by straight arrows. (D) The extended helical structure from TM5 interacts with G11; interface residues are depicted as sticks.
