Abstract
Following initial success in melanoma and lung tumours, immune checkpoint inhibitors (ICIs) are now well recognized as a major immunotherapy treatment modality for multiple types of solid cancers. In colorectal cancer (CRC), the small subset that is mismatch-repair-deficient and microsatellite-instability-high (dMMR/MSI-H) derive benefit from immunotherapy; however, the vast majority of patients with proficient MMR (pMMR) or with microsatellite stable (MSS) CRC do not. Immunoscore and the consensus molecular subtype classifications are promising biomarkers in predicting therapeutic efficacy in selected CRC. In pMRR/MSS CRC, biomarkers are also needed to understand the molecular mechanisms governing immune reactivity and to predict their relationship to treatment. The continuous development of such biomarkers would offer new perspectives and more personalized treatments by targeting oncological options, including ICIs, which modify the tumour-immune microenvironment. In this review, we focus on CRC and discuss the current status of ICIs, the role of biomarkers to predict response to immunotherapy, and the approaches being explored to render pMMR/MSS CRC more immunogenic through the use of combined therapies.
Keywords: colorectal cancer, immunotherapy, immune checkpoint inhibitors, immune response, immunoscore
Introduction
Colorectal cancer (CRC) is the second most common cancer in women and the third most common in men. Despite advances in the diagnosis and management of this disease, CRC remains the fifth cause of cancer-related death in women and the fourth cause in men [1]. Moreover, the global CRC burden is expected to increase by 60% by 2030 [2].
The immune system distinguishes self from non-self through the binding of T-cell receptors (TCR) on T-cells to complexes of peptides with major histocompatibility complex (MHC) class I molecules presented on the surface of all cells, including tumour cells [3, 4]. Recognition of peptide–MHC class I complexes by the TCR alone is insufficient for T-cell activation. TCR–MHC signalling pathways are modulated by co-stimulatory or co-inhibitory signals, which tumour cells exploit to escape destruction [5, 6]. Immune checkpoint inhibitors (ICIs) are a type of immunotherapy often made from antibodies. ICIs target co-inhibitory receptors, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on T-cells and other immune-cell subpopulations, or their ligands, such as programmed cell death protein 1 ligand 1 (PD-L1) on tumour cells and various immune cells. Over the past decade, ICIs have revolutionized the field of oncology through demonstrated clinical efficacy in several cancers, including melanoma and non-small-cell lung cancer. To date, other inhibitory receptors such as lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin (Ig) mucin 3 (TIM-3), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), and activating receptors such as the tumour necrosis factor receptor (TNFR) superfamily, member 4 (OX40), the glucocorticoid-induced TNFR-related protein, the inductible T-cell costimulator, and CD40 have been identified and are currently evaluated as targets of monoclonal antibodies in different clinical trials [7].
In CRC, it has been shown that only patients with the subset of mismatch-repair-deficient or microsatellite instability-high (dMMR/MSI-H) tumours are likely to respond to treatment with ICIs [8–10]. This subset is characterized by an increased number of tumour mutations [11] due to inactivation of one of the four mismatch-repair (MMR) genes: MSH2, MLH1, MSH6, and PMS2 [12–15]. In 12% of CRC cases, epigenetic changes cause sporadic dMMR/MSI-H, in particular methylation of the MLH1 promoter. While, in 3% of CRC cases, dMMR/MSI-H is due to germ-line MMR mutation (Lynch syndrome) [16]. In 2017, the Food and Drug Administration (FDA) approved the anti-PD-1 inhibitors pembrolizumab (Keytruda®, Merck) and nivolumab (Opdivo®, Bristol-Myers Squibb) for the treatment of patients with dMMR/MSI-H CRC, but the European Medicines Agency is still waiting for the results of phase III randomized–controlled studies.
Unlike dMMR/MSI-H CRC patients, ICIs alone provide limited to no clinical benefit in CRC patients with proficient MMR or microsatellite stable (pMMR/MSS) tumours [8]. For these patients, ICIs are being actively explored in combination with treatments that aim to increase the intra-tumoural immune response and render the tumour ‘immune-reactive’. In this review, we discuss the current use of ICIs in CRC, the role of biomarkers to predict CRC response to immunotherapy, and approaches currently under investigation to render pMMR/MSS CRC more immunogenic through the use of combined therapies.
Immunotherapy in CRC: current status
Ipilimumab (Yervoy®, Bristol-Myers Squibb) is a monoclonal antibody that targets the CTLA-4 protein receptor to activate the immune system [17–21]. Its rapid success, and that of monoclonal antibodies against PD-1 and its ligand PD-L1 [22–25], led to the active investigation of ICIs in all cancer types. In the initial trials, which included patients with unselected metastatic CRC (mCRC), only three out of >100 patients with treatment-refractory mCRC experienced a partial or complete response following anti-CTLA-4 or anti-PD-1/PD-L1 treatment [23, 26–28]. Retrospectively, it was found that all responders harboured dMMR/MSI-H tumours. Most of these tumours foster an immunogenic microenvironment characterized by a high overall mutation burden (>12 mutations per 106 DNA bases), associated tumour neoantigens and T helper 1 (Th1) cytotoxic immune response with upregulation of PD-1/PD-L1-positive cells [29–33]. Based on the observed impressive tumour response, enthusiasm for immunotherapy in CRC grew and several studies investigated the therapeutic potential of PD-1 inhibitors.
Le and colleagues reported the results of a phase II proof-of-concept study (KEYNOTE-016) of dMMR/MSI-H tumours treated with pembrolizumab (10 mg/kg every 2 weeks) [8]. In this trial, which included 41 patients with dMMR/MSI-H and pMMR/MSS chemorefractory mCRC and dMMR/MSI-high non-CRC patients, the overall response rate (ORR) was 40% (4 of 10 patients). Clinically durable responses were observed in patients with dMMR/MSI-H mCRC, whereas no response (ORR = 0%) was observed in those with pMMR/MSS mCRC (0/18). Treatment was well tolerated overall, but 17 of 41 patients experienced a grade 3–4 treatment-related adverse event (TRAE). The updated results of this trial, which included 86 dMMR/MSI-H cancers, confirmed an ORR of 53%, with 21% complete responses. In CRC, objective responses were observed in 52% of patients [34]. The 2-year overall survival (OS) rate was 64% for these highly pretreated cancers [34].
CheckMate-142, a multicohort non-randomized phase II study, evaluated the efficacy and safety of nivolumab (3 mg/kg every 2 weeks) in combination with ipilimumab (1 mg/kg every 3 or 6 weeks), or nivolumab as a single agent in previously treated or treatment-naïve dMMR/MSI-H mCRC [9, 10, 35]. The results of this study confirmed the impressive treatment benefit of these drugs in this setting. In chemorefractory mCRC patients, the ORR for nivolumab monotherapy (n = 74) was 31% and for the nivolumab/ipilimumab combination (n = 119) it was 55%; 1-year OS rates were 73.4% and 85%, respectively [9, 10]. In treatment-naïve patients, the corresponding ORR and 1-year OS rates for first-line patients treated with the combination regimen (n = 45) were 60% and 83%, respectively [10]. With >2 years of follow-up at the time of reporting, the median OS of all cohorts had not been reached. Grade 3–4 TRAEs were higher in the combination regimen (32%) except for the cohort that received combined therapy with low-dose ipilimumab (1 mg/kg every 6 weeks). In these patients, grade 3–4 TRAEs were 16%, suggesting that the low-dose combination is the best treatment regimen in this setting. Efficacy of nivolumab and ipilimumab combination was also recently reported in the preoperative setting. In this ongoing study, authors preliminarily reported that a short preoperative treatment (6 weeks) with ipilimumab (1 mg/kg, day 1) plus nivolumab (3 mg/kg, day 1, 15) in non-metastatic colon cancer was safe and led to major pathological response (≤2% of residual vital tumour cells) in all dMMR/MSI-H tumours (seven of seven patients) [36]. No sign of pathological response (85%–100% of residual vital tumour cells) was observed in the eight patients with pMMR/MSS CRC tumours.
Several randomized phase III trials are currently ongoing in dMMR/MSI-H mCRC to evaluate the efficacy of anti-PD-1, anti-PD-L1, and anti-CTLA-4 either combined with or compared to chemotherapy and with or without targeted therapy [37]. However, dMMR/MSI-H status, well recognized for its favourable prognosis in localized CRC [38], results in a lower proportion of patients (3%–4%) with metastatic dMMR/MSI-H CRC [39] who are available to benefit from such trials.
Unlike patients with dMMR/MSI-H mCRC, immunotherapy alone has not demonstrated a clinical benefit in pMMR/MSS mCRC and this subset constitutes most tumours. In the pivotal KEYNOTE-016 study with pembrolizumab, no responses were observed in these patients [8], consistently with the lack of efficacy of immunotherapy in early studies with non-selected patients, most of whom had pMMR/MSS mCRC. In the CheckMate-142 study, limited responses were seen in pMMR/MSS tumours. The lack of CRC immunoreactivity and recruitment of immune cells seems to be the fundamental obstacle to efficacy. Combination treatment with PD-1 inhibitors and modulators of other immune checkpoint molecules, such as CTLA-4, might be beneficial in a subset of patients with pMMR/MSS tumours, as reported in a recent study [40]. Nevertheless, alternative approaches to modulate the tumour-immune microenvironment are currently being explored for the many CRC patients who harbour the pMMR-MSS subtype.
Biomarkers for response to immunotherapy beyond dMMR/MSI status
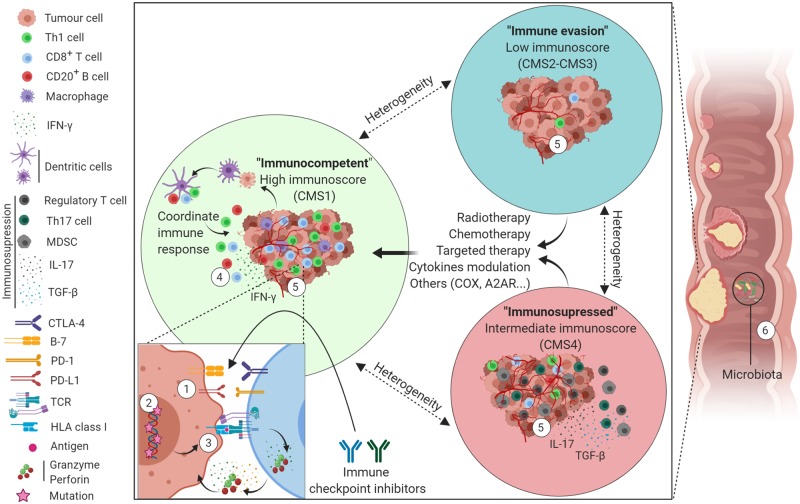
The presence of dMMR/MSI-H in solid tumours, including CRC, is now a clear potential biomarker for response to immunotherapy [7]. However, given the complexity of the antitumour immune response and the intra-tumoural and inter-metastasis heterogeneity, dMMR status alone is presumably not enough to accurately identify responders to ICIs [41]. Identification of more precise and reliable predictive biomarkers continues to be an unmet clinical need. A summary of promising biomarkers discussed hereafter is shown in Figure 1.
Figure 1.
Schematic representation of colorectal-cancer-immune subgroups, linked biomarkers, and potential treatment strategies. ‘Immunocompetent’ tumour (green circle) is characterized by a coordinated immune response with high T-cells (CD3, CD8, and Th1), macrophage infiltration, and upregulation of immune checkpoint molecules (CTLA-4, PD-1, and PD-L1). ‘Immune evasion’ group (blue circle) is characterized by poor immune cell infiltration. ‘Immunosuppressed’ group (red circle) is characterized by high immune cell infiltration as well as a high infiltration of suppressor cells with suppressive cytokine release. The ‘immune evasion’ and the ‘immunosuppressed’ groups could be treated with radiotherapy, chemotherapy, targeted therapy, cytokine modulation, or other approaches such as COX inhibition or A2AR inhibition, to render the tumours more immunogenic so that they benefit from ICIs. Possible biomarkers of response to ICIs are marked from 1 to 6. (1) PD-1/PD-L1 expression. (2) Tumour-mutation burden. (3) Mutation-associated neoantigens presentation on HLA class I. (4) High cytotoxic immune cells infiltration (immunoscore). (5) Gene-expression signature (including CMS classification). (6) Gut microbiome. A2AR, adenosine A2A receptor; COX, cyclooxygenase-2; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; HLA, human leucocyte antigens; IFN-γ, interferon-gamma; IL-17, interleukin 17; MDSC, myeloid-derived suppressor-derived cells; PD-1, programmed death protein 1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor; TGF-β, transforming growth factor-beta; Th17, T helper 17; CMS, consensus molecular subtype.
PD-1/PD-L1 expression
PD-1 is expressed by activated T-cells, B-cells, and natural killer (NK) cells and can bind to its ligand, PD-L1, as expressed on tumour cells [42]. This expression is, in part, induced by interferon-gamma (IFN-γ) that is produced by activated lymphocytes. PD-L1 expression, measured by immunohistochemical staining, has been extensively evaluated as a predictive biomarker of response to ICIs. Interestingly, in some tumour types, such as non-small-cell lung carcinoma (NSCLC), gastric cancer, and oesophageal tumours, PD-L1 expression might be useful as a predictive marker of response to anti-PD-1 therapy [43–46]. However, several issues prevent PD-L1 expression from being a clinically useful biomarker. The use of various PD-L1-detection antibodies, the lack of standardization of PD-L1-expression evaluation, and the percentage of positive cells cut-off (ranging from >1% to >50% of tumour cells stained) limit its statistical significance. Moreover, PD-L1 expression can be induced by IFN-γ or constitutive oncogene activation [47]. PD-L1 expression is a dynamic process that changes according to the tumour microenvironment and disease stage, and it could well be influenced by treatment [48]. PD-L1 expression in tumours is not uniform, and the sampling time and location may affect the results of PD-L1 staining [49]. Finally, patients who respond to ICIs, but do not express PD-L1, limit its clinical impact.
In CRC, PD-L1 expression was poorly correlated with dMMR/MSI-H status [50] and not found to be associated with response or survival in the registration studies [35], thus limiting its impact in this particular type of solid tumour. A recent meta-analysis revealed that PD-L1 expression can serve as a significant biomarker for negative prognosis that is not related to clinicopathological characteristics [51].
Endogenous antitumour T-cell immunity is largely restricted to PD-1-high cytotoxic lymphocytes. Infiltration of NSCLC with such PD-1 high CD8+ T-cells has recently been associated with clinical response to anti-PD-1 [52, 53]. Interestingly, Llosa and colleagues similarly reported that pMMR/MSS tumours infiltrated by PD-1-high CD8+ cytotoxic lymphocytes (without suppressor T helper 17 [Th17] cells) and expressing a high level of PD-L1 have an immune microenvironment resembling that of the dMMR/MSI-H and were associated with pembrolizumab benefit [54]. As previously reported in digestive cancers and melanoma [52], these PD-1-high CD8+ cytotoxic lymphocytes were characterized by an exhausted/memory transcriptome, suggesting the presence of an antitumour T-cell repertoire [54].
Neoantigens and mutational burden
Due to the hypermethylation of MLH1 [55, 56] or mutations in MMR genes [57], dMMR/MSI-H tumours harbour a high frequency of insertions/deletions (indels) in microsatellite sequences [58] and a high tumour mutational burden (TMB) [59] that result in a high mutation-associated neoantigen (MANA) load [29–31]. These neoantigens can be processed and presented by dendritic cells leading to the priming of a coordinated adaptive anticancer immune response [32], which explains the higher density of tumour-infiltrating lymphocytes (TILs) and activated Th1 cells, as well as increased type I interferon production, observed in these tumours. This tumour-immune surveillance leads to the immunoediting concept. Three essential phases have been proposed: elimination, equilibrium, and escape [60]. During the elimination, innate and adaptive coordinate immune responses act together for the successful eradication of tumour cells. During the equilibrium phase, the continuous immune-selection pressure also induces a Darwinian selection process in which new tumour variants emerge, carrying various genetic and epigenetic changes in tumour behaviour such as the upregulation of checkpoint receptors including CTLA-4, PD-1, PD-L1, indolamine 2, 3-dioxygenase 1 (IDO1), and LAG-3 [33], or the downregulation of antigen-presenting molecules. During the escape phase, various tumour-derived soluble factors contribute to the induction of a local immunosuppressive environment favouring immune escape, progression, and metastasis [61, 62].
In CRC, 12.8% of tumours simultaneously present TMB-high, dMMR-MSI-H, and PD-L1 expression [50]. A high percentage of concordance (44.2%) of TMB-high and dMMR-MSI-H was observed [50] and was predictive of response to ICIs [40, 63]. However, PD-L1 expression demonstrates great variability and can be expressed even in dMMR-MSI-H-negative and/or TMB-low cases. Interestingly, it is possible to have a TMB-high in the absence of dMMR-MSI-H, whereas dMMR-MSI-H with TMB-low is rare [50]. There is the need to standardize PD-1/PD-L1 expression and define cut-offs for TMB interpretation. The development of a liquid-biopsy method to identify tumours with dMMR-MSI-H or TMB-high is a non-invasive technique demonstrating its efficacy for the determination of response to ICIs [64].
Additionally, tumours harbouring mutations in the region encoding for the POLE exonuclease domain also presented high TMB and MANA load [65, 66]. POLE mutations are present in 1%–2% of all CRC tumours. Similarly to dMMR/MSI-H CRC, patients with POLE-mutated CRC have a good prognosis, resulting in a lower proportion of mCRC than in stage I–III CRC [67]. They also present a higher level of CD8+ lymphocyte infiltration, expression of cytotoxic T-cell markers, and effector cytokines [68]. Given the similarly enhanced immunogenicity of POLE-mutated CRCs to dMMR/MSI-H CRCs, the therapeutic potential of immune checkpoint blockade in the subset of POLE-mutated CRCs is of particular interest [69]. Further investigation is currently underway in clinical trials. Therefore, a high TMB and MANA load, associated with a high T-lymphocyte infiltration, and T-cell diversity [70–72] have emerged as biomarkers of response to ICIs in several tumour types [73, 74].
Immune infiltration (immunoscore)
Interestingly, a high TMB might not always be necessary to drive an immune response. Evaluation of the presence of tumour-infiltrating CD3+ and CD8+ lymphocytes, through assignment of an immunoscore based on the density and the location of subsets of T-cells, was prognostic of clinical outcome in patients with stage I–III CRC and performed better than MSI and MMR status [75–77]. The immunoscore is a simple scoring system based on the numeration of two lymphocyte populations (CD3/CD45RO, CD3/CD8, or CD8/CD45RO), both in the core of the tumour and in the invasive margin of tumours. The immunoscore provides a score ranging from Immunoscore 0 (I0) when low densities of both cell types are found in both regions to Immunoscore 4 (I4) when high densities are found in both regions [78].
Recently, the prognostic impact of the immunoscore was validated in a study with samples from 2,681 stage I–III colon cancer patients from 14 centres in 13 countries [77]. Interestingly, the authors showed that patients with a high immunoscore had similar tumour relapse and survival, and that this was independent of their dMMR/MSI-H status. Therefore, it is possible to conclude that the favourable prognosis observed in dMMR/MSI-H-localized CRC is essentially related to high immune infiltration. Furthermore, the prognostic value of the immunoscore was validated in a meta-analysis of eight studies published between 2011 and 2018 [79], supporting the idea that TILs play an important role in disease control across all disease stages by preventing the dissemination of metastasis to lymph nodes and organs.
In mCRC characterized by multiple tumour lesions among different organs, the picture is more complex. We previously reported a comprehensive analysis of patients having undergone complete resection of all metastases and revealed the heterogeneity of the metastatic disease and its clinical impact [80–83]. Complex tumour-immune interrelations shape the metastatic landscape, not only in terms of lesion size, number, or mutational pattern, but also in terms of immune-cell infiltration. We observed a heterogeneous immune infiltrate, immunoscore, and mutational diversity within the multiple resected synchronous and metachronous metastases of patients. Adaptive immune cells and immunoscore quantified in the least-infiltrated metastasis per patient were the most associated with patient long-term survival. Within any specific patient, a high immune infiltrate/high immunoscore correlated with fewer metastases and improved long-term survival [80, 81]. In patients with unresectable mCRC, where adequate immunoscore assessment is impossible, we reported that a single biopsy of a metastasis was able to accurately identify low-infiltrated metastases, but that the overall intra-metastatic immune infiltrate might be better estimated with multiple biopsies or the sampling of larger tumour areas [81]. Importantly, on biopsies, the performance of the immunoscore was superior to that of PD-L1 in estimating the reality of concordance across the whole metastatic slide [80, 81]. This illustrated the fact that PD-L1 stainings were more heterogeneous in a given metastasis.
High immunoscores reported in pMMR/MSS CRCs raise the question of whether immunophenotyping might predict which patients will benefit from immunotherapy. Just recently, some authors reported that higher CD3+ and CD8+ T-cell densities (but not PD-L1 expression on cancer cells) were associated with a higher ORR and duration of disease control in a small subgroup of patients with dMMR/MSI-H mCRC treated with pembrolizumab [84]. This suggests that a lower immunoscore is associated with immunotherapy resistance in dMMR/MSI-H tumours, which is consistent with the reported finding that lower immunoscores are associated with worse survival in patients with dMMR/MSI-H stage III colon cancers receiving cytotoxic chemotherapy [85]. Hence, combining the immunogenic features of the tumour microenvironment with TMB may be more precise in predicting immunotherapy response than either feature alone. Further validation of immunophenotyping as a predictive biomarker of immunotherapy response, specifically in metastatic disease, is needed for broad clinical utility and is currently being explored in our trials (NCT03608046 and NCT03127007).
Gene-expression signature
A comprehensive re-evaluation and comparison of CRC molecular gene-expression profiles has enabled the CRC Subtyping Consortium (CRCSC) to identify four robust consensus molecular subtype (CMS) classifications. The first type, CMS1—immune, is mainly composed of dMMR/MSI-H tumours and is characterized by a high TMB, high immune infiltration and activation, and BRAF mutations. The second type, CMS2—canonical, is characterized by WNT and MYC activation. The third, CMS3—metabolic, is characterized by cancer-cell metabolic deregulation and KRAS mutation. The fourth type, CMS4—mesenchymal, is characterized by stromal infiltration, transforming growth factor-beta (TGF-β) activation, and angiogenesis [65]. Interestingly, the immune microenvironment of each CMS type is different. Stage-independent prognostic values and significant associations with clinical, biological, and treatment features have been demonstrated and recently validated in phase III clinical studies [86–88].
CMS1 and CMS4 are both ‘hot’ tumours; they are considered to be immune-reactive and highly infiltrated by immune cells as opposed to CMS2 and CMS3, which are ‘cold’ tumours. Despite CMS1 and CMS4 being immune-reactive, they each present distinct immune features and escape mechanisms. CMS1 present CD8+ T-cells and CD68+ macrophage infiltration as well as frequent upregulation of immune checkpoint molecules (CTLA-4, PD-1, and PD-L1) responsible for the main immune escape mechanism in these tumours [89]. CMS4 present a different pattern of immune infiltration, which is mainly suppressive throughout the infiltration of myeloid-derived suppressor cells (MDSCs), T-regulatory cells (Tregs), monocyte-derived cells, and Th17 cells. In these tumours, immunosuppressive factors such as TGF-β and CXCL12 are upregulated along with chemokines, such as interleukin 23 (IL-23) and interleukin 17 (IL-17) [90]. CMS1 and CMS4 tumours are therefore likely to respond well to immune therapies, but they should each be treated distinctly. Patients harbouring CMS1 tumours could theoretically benefit from ICIs alone, whereas those with the CMS4 subtype would be best suited to strategies combining TGF-β inhibitors, Tregs, MDSCs inhibition, and ICIs. The CMS classification is a promising new biomarker of response to immune therapies; however, as is the case with other biomarkers, care should be taken when selecting biopsies and resection specimens for CMS classification. It should be borne in mind that CMS classification was mainly derived from samples of primary non-metastatic CRC (92% of the samples); it was not totally reproducible on metastatic samples (e.g. liver metastases) [91] or validated in the metastatic setting [86]. Additionally, spatial- and temporal-tumour heterogeneity, predominant in the metastatic setting, can misevaluate CMS status. Thus, the source of the sample and prior treatments before collection must be carefully considered.
A recent study described an innate immune response in some CRC as being due to the upregulation of PD-L1 and IDO1 linked to DNA damage. In order to identify this subtype of tumours with defective DNA-damage response (DDR), the authors developed a 44-gene signature assay and reported that 80% of dMMR/MSI-H and 25% of pMMR/MSS tumours presented the signature. The DDR assay could therefore enable the identification of not only dMMR/MSI-H tumours, but also rare cases of pMMR/MSS tumours likely to respond to ICIs [92].
Microbiota
The gut microbiome also influences the outcome of cancer therapy by modulating the host inflammatory response [93, 94]. An intact microbiome is required for successful tumour control in response to genotoxic (e.g. oxaliplatin used in CRC) and immunomodulatory therapies (e.g. cyclophosphamide). Recent studies have reported the important role of the gut microbiome with ICI treatment [95–97]. It was found that primary resistance to ICIs (in melanoma and lung cancers) could be attributed to an abnormal gut microbiome due to the use of antibiotics. One study raises the hypothesis that transplanting faecal material from responding patients to non-responders could lead to improved tumour control. In this study, it was shown that, when germ-free mice were transplanted with faecal material from melanoma patients, they experienced improved tumour control, augmented T-cell responses, and greater efficacy of anti-PD-LI therapy [98]. Metagenomics of patient stool samples at diagnosis have also revealed correlations between clinical responses to ICIs and the relative abundance of Akkermansia muciniphila [95]. This influence of the microbiome in the outcome of cancer treatment and the function of anticancer immunity poses new questions from a preclinical and clinical standpoint in the CRC field. Despite some evidence of an association between the gut microbiome and CRC, its role in the treatment of advanced and metastatic CRC remains largely unexplored.
Immunotherapy for pMMR/MSS CRC: strategies and development
The recent success of anti-PD-1 and anti-CTLA-4 treatments in dMMR/MSI-H patients and preclinical data showing that dMMR/MSI-H tumours are not the only subgroup of CRC that could, theoretically, benefit from ICIs have led to the design of new clinical studies. These trials aim to select the subset of CRC patients most likely to respond to ICIs or to design novel therapeutic strategies to render these tumours ‘immune-competent’ (Figure 1). Some oncological treatments may also be able to cause immunogenic cell death (ICD)—a form of cell apoptosis that can induce an antitumour immune response and potentially overcome the primary resistance of pMMR/MSS CRC to ICIs. The main treatment strategies currently being investigated in clinical trials are summarized in this section and in Table 1.
Table 1.
Selection of ongoing trials investigating different treatment strategies with ICIs for pMMR-MSS CRC
| Combined therapy | Target | Clinical compound | ICI compound | Trial type | Trial identifier |
|---|---|---|---|---|---|
| Radiotherapy | Stereotactic body radiation | Toripalimab | Phase IImCRC with oligometastasis | NCT03927898 | |
| Liver radiation therapy | Nivolumab, Ipilimumab, and CMP-001 | Phase ImCRC | NCT03507699 | ||
| Radiation therapy | Nivolumab + Ipilimumab | Phase IIpMMR-MSS and dMMR-MSI CRC | NCT03104439 | ||
| Radio-chemotherapy | 5-FU | Atezolizumab | Phase I/IILocalized rectal cancer | NCT03127007 | |
| Radiation therapy | Durvalumab ± Tremelimumab | Phase IImCRC | NCT02888743 | ||
| Radiation therapyOr ablation | Durvalumab ± Tremelimumab | Phase IImCRC | NCT03122509 | ||
| Radiation therapy | Durvalumab ± Tremelimumab | Phase IImCRC pMMR-MSS | NCT03007407 | ||
| Radio-chemotherapy | Capecitabine | Nivolumab | Phase I/IIRectal cancer | NCT02948348 | |
| Radio-chemotherapy | Standard Radio-chemotherapy | Durvalumab | Phase IIRectal cancer pMMR-MSS | NCT03102047 | |
| Targeted therapies | VEGFR and KIT | Cediranib | Durvalumab | Phase I/IIRefractory CRC | NCT02484404 |
| EGFR | Panitumumab | Nivolumab ± Ipilimumab | Phase IIRAS-wild-type CRC | NCT03442569 | |
| VEGFR, PDGFR, FGFR | Nintedanib | Pembrolizumab | Phase I/IImCRC | NCT02856425 | |
| EGFR | Cetuximab | Pembrolizumab | Phase Ib/IIPretreated mCRC | NCT02713373 | |
| EGFR | Cetuximab + Irinotecan | Avelumab | Phase IImCRC pMMR-MSS | NCT03608046 | |
| EGFR | Cetuximab + FOLFOX | Avelumab | Phase IIUntreated mCRC | NCT03174405 | |
| EGFR | Cetuximab | Avelumab | Phase IIPretreated RAS-wild-type mCRC | CAVE Colon | |
| VEGFA | Bevacizumab + Capecitabine | Atezolizumab | Randomized phase IIRefractory CRC | NCT02873195 | |
| VEGFA | Bevacizumab + FOLFOX | PDR001 | Phase IFirst-line mCRC | NCT03176264 | |
| VEGFA | Bevacizumab + FOLFOX | Nivolumab | Phase II/IIFirst-line CRC | NCT03414983 | |
| VEGFA | Bevacizumab + Capecitabine | Pembrolizumab | Phase IIPretreated mCRC | NCT03396926 | |
| Multikinase | Regorafenib | PDR001 | Phase IbPretreated mCRC | NCT03081494 | |
| MEK | Combimetinib and Regorafenib | Atezolizumab | Phase IIImCRC | NCT02788279 | |
| MEK | Cobimetinib | Atezolizumab | Phase IIFirst-line mCRC | NCT02291289 | |
| MEK | Binimetinib | Nivolumab ± Ipilimumab | Phase I/IIPretreated mCRC | NCT03271047 | |
| MEK | Binimetinib+ FOLFOX or FOLFIRI | Pembrolizumab | Phase IbmCRC | NCT03374254 | |
| MEK | Trametinib | Nivolumab ± Ipilimumab | Phase I/IIPretreated mCRC | NCT03377361 | |
| MEK | Trametinib | Durvalumab | Phase IImCRC pMMR-MSS | NCT03428126 | |
| MEK and VEGFA | Combimetiniband Bevacizumab | Atezolizumab | Phase ImCRC | NCT02876224 | |
| MEK, CD38, LAG-3 | Cobimetinib, Daratumumab, anti-LAG-3 antibody | Nivolumab ± Ipilimumab | Phase IIRefractory CRC | NCT02060188 | |
| PI3K | Copanlisib | Nivolumab | Phase I/IIUnresectable or mCRC pMMR-MSS | NCT03711058 | |
| MNK | eFT508 | Avelumab | Phase IIRelapsed or refractory pMMR-MSS | NCT03258398 | |
| Cytokines | IL-15 superagonist | ALT-803 | Pembrolizumab, or Nivolumab, or Atezolizumab, or Avelumab | Phase IIAdvanced cancer including CRC | NCT03228667 |
| CXCL12 | Olaptesed pegol | Pembrolizumab | Phase I/IImCRC | NCT03168139 | |
| Cytokines release | Poly-ICLC | Pembrolizumab | Phase I/IImCRC | NCT02834052 | |
| GM-CSF | talimogene laherparepvec | Atezolizumab | Phase ImCRC | NCT03256344 | |
| CSF-1R | Pexidartinib | Durvalumab | Phase ICRC | NCT02777710 | |
| Others | COX-2 | Celecoxib | Nivolumab ± Ipilimumab | Phase IIStage I–III CRC | NCT03026140 |
| IDO1 | Epacadostat | Nivolumab | Phase I/IISolid tumours including CRC | NCT02327078 | |
| IDO1 and DNMT | Epacadostat and Azacitidine | Pembrolizumab | Phase I/IIRefractory CRC and NSCLC | NCT02959437 | |
| DNMT | Azacitidine | Durvalumab | Phase IImCRC | NCT02811497 | |
| DNMT and HDAC | Azacitidine and romidepsin | Pembrolizumab | Phase IPretreated mCRC | NCT02512172 | |
| Thymidine phosphorylase | TAS-102 | Nivolumab | Phase IIRefractory CRC | NCT02860546 | |
| Thymidine phosphorylaseVEGFA | TAS-102Bevacizumab and Capecitabine | Nivolumab | Phase IIPretreated mCRC | NCT02848443 | |
| Glucose metabolism | Metformin | Nivolumab | Phase IIRefractory pMMR-MMS CRC | NCT03800602 | |
| Adenosine receptor | AZD4635 | Durvalumab | Phase ISolid malignancies including CRC | NCT02740985 | |
| Adenosine receptor | NIR178 | PDR001 | Phase IIAdvanced solid tumours including CRC | NCT03207867 | |
| Adenosine receptorCD73 | NIR178NZV930 | PDR001 | Phase IAdvanced solid tumours including CRC | NCT03549000 | |
| EGFR-CAR T-cells expressing anti-PD-1 and anti-CTLA-4 antibodies | Phase I/IIEGFR positive advanced malignant solid tumours | NCT03182816 | |||
| EGFR-CAR T-cells expressing anti-PD-1 antibodies | Phase I/IIEGFR positive advanced malignant solid tumours | NCT02873390 | |||
| MUC1-CAR T-cells expressing anti-PD-1 and anti-CTLA-4 antibodies | Phase I/IIMUC1 positive advanced malignant solid tumours | NCT03179007 |
Clinical trial details can be accessed at ClinicalTrials.gov.
5-FU, 5-fluorouracil; Avelumab, anti-PD-L1; Atezolizumab, anti-PD-L1; CMP-001, anti-TLR9; CAR, chimeric antigen receptor; CRC, colorectal cancer; CSF-1R, colony stimulating factor 1 receptor; COX-2, cyclooxygenase-2; CXCL12, C-X-C motif chemokine 12; DNMT, DNA methyltransferase; Durvalumab, anti-PD-L1; dMMR/MSI, mismatch-repair-deficient and microsatellite instable; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FOLFIRI, 5-fluorouracil, leucovorin, and irinotecan; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; GM-CSF, granulocyte-macrophage colony stimulating factor; HDAC, histone deacetylase; IDO1, indolamine 2.3-dioxygenase 1; IL-15, interleukin 15; Ipilimumab, anti-CTLA-4; KIT, tyrosine kinase Kit; LAG-3, lymphocyte-activation gene 3; mCRC, metastatic colorectal cancer; MEK, mitogen-activated protein kinase; MNK, mitogen-activated protein kinase interacting protein kinase; MUC1, mucin-1; Nivolumab, anti-PD-1; NSCLC, non-small-cell lung cancer; PDGFR, platelet-derived growth factor receptor; PDR001, anti-PD-1; Pembrolizumab, anti-PD-1; pMMR/MSS, mismatch-repair-proficient and microsatellite stable; Toripalimab, anti-PD-1; Tremelimumab, anti-CTLA-4; VEGFA, vascular endothelial growth factor A; VEGFR, vascular endothelial growth factor receptor.
Radiation therapy
Preclinical and early clinical studies have suggested that radiation therapy (RT) or chemo-radiation therapy (CRT) may be a clever way to expose neoantigens. By damaging DNA, radiotherapy induces tumour-cell death. This releases neoantigens creating immune-mediated antitumour responses [99] and ICD [100, 101]. In some patients, this ‘neoantigens release’ can act as an in situ radiation-induced vaccine [102, 103]. This immune effect is applicable not only to the irradiated tumour site, but also to distant sites through the ‘abscopal effect’ [104], which theoretically could be enhanced with ICIs. It has been shown, for example, that anti-CTLA-4 and/or anti-PD-L1 in combination with RT can work synergistically to significantly improve treatment response (including metastatic lesions outside the radiation field) and patient outcomes in metastatic melanoma and NSCLC [104, 105].
The data to understand the role of RT and ICIs in CRC are, however, limited. In mCRC, stereotactic body radiation therapy (SBRT) directed to a site of liver metastasis in combination with a PD-1 inhibitor did not illicit any response [106]. In another trial in mCRC evaluating pembrolizumab with palliative RT or with local ablation, one major response (1/11 patients, ORR = 9%) in a metastatic site distant from the irradiated field was observed in the RT cohort [107]. Recently, dual blockade of CTLA-4 (ipilimumab) and PD-1 (nivolumab) with RT (8 Gy in three fractions to a single metastatic lesion) demonstrated feasibility and promising activity in a phase II study that included 40 patients with chemorefractory mCRC [108]. Among the 27 patients treated with protocol-defined RT (33% of patients never received RT due to progression or grade 3–4 TRAE), the ORR and disease-control rate (DCR) were 15% and 37%, respectively. The durability of disease control was impressive, with a median of >15 months. Modification of the dosing schedule to reduce the dropout rate should be considered for future development. The outcome of correlative studies (biopsies, whole-exome sequencing) are eagerly awaited.
Several clinical trials evaluating the efficacy of conventional or stereotactic RT in combination with ICIs are ongoing (Table 1). Our research group is currently conducting a multicentre randomized phase II trial (R-Immune trial, NCT03127007) evaluating the benefit of atezolizumab (anti-PD-L1) with preoperative CRT (45–50 Gy over 5 weeks in combination with 5-fluorouracil) for locally advanced rectal cancer. The trial design delays atezolizumab administration until 2 weeks after CRT initiation in order to explore the role of CRT alone on the tumour-immune microenvironment. The trial also incorporates multiple tumour, blood, and stool collections to investigate the role of several biomarkers (as previously described) in multiple correlative sub-studies.
Chemotherapy and targeted therapies
The first two lines of treatment in mCRC currently involve a combination of targeted therapies that inhibit the epidermal growth factor receptor (EGFR; cetuximab and panitumumab), or angiogenesis (bevacizumab, aflibercept, or ramucirumab), together with chemotherapy (5-fluorouracil, irinotecan, oxaliplatin) [109–113]. Recent evidence suggests that these chemotherapy regimens can induce ICD by releasing damage-associated molecular patterns (DAMPs) [114, 115] and activating necrotic or apoptotic pathways [116]. The translocation of calreticulin is then recognized as a signal by dendritic cells (DCs) to mediate the phagocytosis of dying tumour cells. Agents such as 5-fluorouracil can induce the apoptosis of MDSCs, therefore inhibiting their immunosuppressive function and increasing CD8+ T-cell function [117]. The analysis of the immune microenvironment of resected CRC liver metastases revealed that patients treated with preoperative chemotherapy had a significantly higher density of cytotoxic and memory T-cells compared with metastases of untreated patients [80, 81, 118]. Moreover, CRC liver metastases, which had achieved pathological and radiological responses, were associated with a significantly higher immunoscore, reflecting an increased adaptive immune response [81].
Vascular endothelial growth factor (VEGF) is a signal protein that stimulates the formation of blood vessels. It is often upregulated in cancer and contributes to tumour angiogenesis [119]. It also plays a role in the immune microenvironment by upregulating immune checkpoint molecules (PD-1, PD-L1, CTLA-4, LAG-3) and downregulating antigen-presentation molecules. Additionally, VEGF inhibits DC maturation and increases the function of suppressor cells [120–128]. Studies combining antiangiogenic agents with ICIs, with or without chemotherapy (FOLFOX), initially suggested potentially synergistic activity [129, 130]; however, the randomized phase III MODUL trial failed to confirm the preliminary findings. In this trial, maintenance treatment with combined atezolizumab/bevacizumab/fluoropyrimidine after first-line induction with FOLFOX/bevacizumab did not demonstrate any clinical benefit in progression-free survival (PFS) or OS compared to bevacizumab and fluoropyrimidine alone.
Preclinical data have shown that cetuximab (a chimeric immunoglobulin G1 [IgG1] monoclonal antibody directed to the EGFR) combined with FOLFIRI (a combination chemotherapy regimen that does not induce ICD) induces ICD in a mouse model and CRC cell lines [131] and may favour the activation of T-cell-mediated immune response [128]. Cetuximab can also stimulate NK-mediated cell-antibody-dependent cellular cytotoxicity [132]. We previously reported that patients treated preoperatively with chemotherapy and cetuximab had a higher infiltration of global (CD3+), cytotoxic (CD8+), and memory (CD45RO+) T-cells into the core of their resected CRC liver metastases and higher immune-related gene expression compared to other treatments [80, 81]. This modification of the tumour-immune microenvironment has also been reported by another group [133] and observed in the small subgroup of patients with RAS-mutated mCRC [80]. This suggests that the immunological effect of cetuximab, unlike its cytotoxic activity, is independent of RAS-mutation status [80]. This promising treatment approach is currently being evaluated in a phase II trial combining avelumab (anti-PD-L1) with FOLFOX and cetuximab in first-line RAS and BRAF wild-type (wt) mCRC (AVETUX trial). The preliminary results from 20 patients have been reported demonstrating an ORR of 75% and a DCR of 95% [134]. Another multicentre phase II trial is currently investigating the question of cetuximab in combination with avelumab as a rechallenge in mCRC patients who have already experienced a partial or complete response with an anti-EGFR plus chemotherapy in first-line treatment (CAVE Colon study). In the same way, our group is currently investigating the efficacy of avelumab combined with cetuximab and irinotecan (AVETUXIRI trial, NCT03608046) in mCRC patients refractory to chemotherapy (cohort A: RAS-mutated) and anti-EGFR treatment (cohort B: RAS wt). We hope to confirm that the immunological effect of cetuximab can provoke a tumour response when combined with avelumab irrespective of RAS mutation. We also plan to prospectively investigate the previously described efficacy biomarkers. For this reason, multiple tumour biopsies and blood collection are planned in the correlative sub-study.
Preclinical studies have reported that MEK inhibitors may be associated with a tumour immunological effect [135, 136]. MEK inhibition upregulates MHC class I expression [137], which promotes antigen presentation on the surface of tumour cells for recognition by CD8+ T-lymphocytes, which then recognize and kill tumour cells. In mouse models, the combination of PD-1 and MEK inhibitors has synergistic tumour-growth inhibition compared to single-agent treatment [135, 136]. This efficacy was clinically observed in a phase Ib study combining atezolizumab (anti-PD-L1) and cobimetinib (anti-MEK) in 23 chemorefractory mCRC patients with an ORR of 17% (4 of 23 patients) [129]. The IMblaze370 (Cotezo) randomized phase III trial failed, however, to confirm this efficacy. Chemorefractory mCRC patients randomized in the combined experimental arm (cobimetinib/atezolizumab) did not experience any increased tumour response or survival benefit (PFS, OS) compared to patients treated with atezolizumab alone or regorafenib [138].
The PI3K-AKT-mTOR pathway is implicated in cell survival, migration, and proliferation, and is often dysregulated in cancer [139]. Interestingly, recent observation suggests that inhibition of this pathway could have not only an effect on tumour cells, but also an effect on the immune microenvironment by preventing the activation of the immunosuppressive pathway [140]. The strategy of combining a PI3K inhibitor and an ICI may lead to an increased ICI response in tumours with an immunosuppressive environment, such as pMMR/MSS CRC. Ongoing trials evaluating chemo and targeted therapies are summarized in Table 1.
Cytokines
Cytokines and chemokines are immune-system molecular messengers. Therefore, targeting these molecules in combination with ICIs is another approach currently under investigation [141].
Interleukin 15 (IL-15) is a glucoprotein that belongs to the 4-alpha-helix bundle family of cytokines. In the immune system, IL-15 is mainly expressed by monocytes, macrophages, and DCs, and has been characterized as a T-cell growth factor [142]. Upon binding to its receptor, highly expressed on CD8+ and NK cells, IL-15 promotes the proliferation and function of these cells. The efficacy of IL-15 administration is limited by its short half-life in vivo [143], but a chimeric fusion protein (ALT-803) that increases the in vivo half-life has been developed. This treatment is currently being tested in combination with different ICIs in patients with advanced cancer, including mCRC patients (NCT03228667).
CXCL12, a chemokine mainly expressed by cancer-associated fibroblasts, has been reported to mediate immune exclusion in mouse models [144]. Therefore, the idea of blocking CXCL12 and the binding to its receptors, CXCR4 and CXCR7, is an interesting novel approach to increase the efficacy of ICIs in immune-excluded tumours with a low immunoscore. This approach is being investigated in a study combining olaptesed (NOX-A12)—a heptamer that binds CXCL12 with high affinity—with pembrolizumab (NCT03168139).
Another way to potentially increase efficacy is to stimulate cytokine release. A phase I/II trial is evaluating how the combination of pembrolizumab and poly-ICLC—a molecule that stimulates cytokine release by inducing IFN-γ production—generates an inflammatory response (NCT02834052).
Even if its function in cancer is controversial [145], human cytokine granulocyte-macrophage colony stimulating factor (GM-CSF) is known to regulate cell differentiation [146] and local recruitment of dendritic cells [147]. This may enhance tumour-associated antigen presentation to T-cells [148] and activate other effectors of the immune response, including macrophages and NK cells [147, 149]. A trial is evaluating a virus, talimogene laherparepvec, that encodes the immunostimulating factor GM-CSF. This virus infects and replicates in tumour cells, leading to cell lysis and GM-CSF release. Combining the talimogene laherparepvec virus with atezolizumab is therefore a promising approach to increase the antitumour immune response in mCRC (NCT03256344).
Others
Other trials underway aim to combine ICIs with molecules that target metabolic pathways. Two trials are currently testing nivolumab with or without ipilimumab combined with celecoxib, a COX-2 inhibitor (NCT03926338 and NCT03026140). The expression of COX-2 may induce expression of IDO1 [150]. IDO1 is an intracellular enzyme that catalyses tryptophan along the kynurenine pathway [151] and induces depletion of tryptophan, leading to an immunosuppressive environment [152, 153]. Higher IDO1 expression in CRC tumours correlates with progressive disease and impaired clinical outcome [154]. For this reason, inhibitors of IDO1, such as epacadostat, are currently being tested in combination with ICIs (NCT02327078 and NCT02959437).
One trial is assessing the combination of metformin with nivolumab (NCT03800602) based on its preliminary reported efficacy in metastatic melanoma [155].
The activation of the A2a and A2b adenosine receptors on immune cells inhibits their proliferation and activation resulting in strong immunosuppression and T-cell anergy [156, 157]. Adenosine has been found to be one of the mechanisms used by Tregs to maintain immunotolerance [158]. Inhibition of the adenosine A2a receptor may potentiate ICIs, such as durvalumab (anti-PD-L1), and could prove to be a worthwhile treatment approach. This strategy is currently being evaluated in a safety trial in patients with advanced solid malignancies, including CRC (NCT02740985).
Cibisatamab (CEA CD3 TCB; RG7802, RO6958688) is a T-cell bispecific antibody (TCB) that simultaneously binds to carcinoembryonic antigen (CEA) on tumour cells and CD3 on T-cells, thus cross-linking cancer cells and T-cells. This leads to T-cell engagement and activation independently of pre-existing immunity, T-cell infiltration, and tumour inflammation. In ongoing studies, encouraging clinical activity has been reported in patients with metastatic pMMR/MSS CRC treated with CEA-TCB monotherapy, and its activity was enhanced when combined with atezolizumab [159]. In the combination therapy group, the ORR was 18% (n = 2) and the observed DCR was 82%. Toxic effects were manageable. CEA-TCB is the first T-cell bispecific antibody to show efficacy in solid tumours and specifically in pMMR-MSS CRC.
Finally, adoptive cell-based immunotherapy with genetically modified T-cells represents a promising emerging modality for CRC treatment. Adoptive cell therapy is based on collection of T-cells from patients, in vitro expansion, and transfusion of T-cells into patients. These T-cells can be engineered to express chimeric antigens receptors (CARs) or selected for their ability to bind tumour antigens. Moreover, CAR T-cells can be engineered to not only recognize tumour antigens, but also to produce cytokines or ICIs. However, despite the fact that CAR T-cells are successfully used for treating haematological cancers such as B-cell malignancies, the efficacy and applicability of cell-based immunotherapy remain to be proved in CRC and other solid tumours [7, 160]. Nevertheless, EGFR-CAR-T or Mucin-1 (MUC1)-CAR T-cells expressing anti-PD-1 or anti-PD-1 and anti-CTLA-4 are currently clinically tested on solid tumours expressing these antigens (EGFR and MUC1).
Conclusion
It is undeniable that considerable advances have been made with the recent FDA approval of ICIs for the treatment of dMMR/MSI-H mCRC. Unfortunately, dMMR/MSI-H CRC represent only a small subgroup of all CRC and most pMMR/MSS mCRC does not benefit from ICIs alone.
Beyond the dMMR/MSI-H tumour status, the continuous development of new biomarkers, such as immunoscore and CMS classification, has led to a better understanding of the molecular mechanisms that define the immune reactivity of CRC and their relationship with oncological treatments. This provides new perspectives, enables a more personalized approach towards patient management, and should continue to be investigated in the translational aspect of clinical trials. That said, many of these biomarkers are governed by a heterogeneous expression pattern in time and space. Therefore, the source of the tumour sample, and any treatments administered prior to sample collection, must be carefully considered.
The key remaining challenge is to identify, among the heterogeneous spectrum of mCRC, which patients are most likely to benefit from ICIs alone or in combination with other oncological treatments, because of their specific genomic and immune tumour characteristics. This question is currently under investigation in ongoing clinical trials.
Acknowledgements
The authors wish to thank Aileen Eiszele for English-language and writing assistance. The Figure 1 has been created with Biorender.com.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
N.H. is a research fellow supported by a grant from the Belgian National Fund for Scientific Research [Télévie/FNRS 7460918F].
References
- 1. Ferlay J, Colombet M, Soerjomataram I. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Sierra MS, Laversanne M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- 3. Khalil DN, Smith EL, Brentjens RJ. et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016;13:273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schreiber RD, Old LJ, Smyth MJ.. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 5. Sharma P, Allison JP.. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 6. Townsend SE, Allison JP.. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 1993;259:368–70. [DOI] [PubMed] [Google Scholar]
- 7. Ganesh K, Stadler ZK, Cercek A. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le D, Uram J, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Overman MJ, McDermott R, Leach JL. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overman MJ, Lonardi S, Wong KYM. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. JCO 2018;36:773–9. [DOI] [PubMed] [Google Scholar]
- 11. Vogelstein B, Papadopoulos N, Velculescu VE. et al. Cancer genome landscapes. Science 2013;339:1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aaltonen LA, Peltomaki P, Leach FS. et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812–6. [DOI] [PubMed] [Google Scholar]
- 13. Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 2006;7:335–46. [DOI] [PubMed] [Google Scholar]
- 14. Strand M, Prolla TA, Liskay RM. et al. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 1993;365:274–6. [DOI] [PubMed] [Google Scholar]
- 15. Ionov Y, Peinado MA, Malkhosyan S. et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558–61. [DOI] [PubMed] [Google Scholar]
- 16. Gupta R, Sinha S, Paul RN.. The impact of microsatellite stability status in colorectal cancer. Curr Probl Cancer 2018;42:548–59. [DOI] [PubMed] [Google Scholar]
- 17. Weber JS, O’Day S, Urba W. et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. JCO 2008;26:5950–6. [DOI] [PubMed] [Google Scholar]
- 18. Yang JC, Hughes M, Kammula U. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwon ED, Drake CG, Scher HI. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carthon BC, Wolchok JD, Yuan J. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16:2861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodi FS, Butler M, Oble DA. et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topalian SL, Sznol M, McDermott DF. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. JCO 2014;32:1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmer JR, Tykodi SS, Chow LQ. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powles T, Eder JP, Fine GD. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. [DOI] [PubMed] [Google Scholar]
- 25. Ansell SM, Lesokhin AM, Borrello I. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung KY, Gore I, Fong L. et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. JCO 2010;28:3485–90. [DOI] [PubMed] [Google Scholar]
- 27. O'Neil BH, Wallmark JM, Lorente D. et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One 2017;12:e0189848.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipson EJ, Sharfman WH, Drake CG. et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 2013;19:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linnebacher M, Gebert J, Rudy W. et al. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 2001;93:6–11. [DOI] [PubMed] [Google Scholar]
- 30. Saeterdal I, Bjorheim J, Lislerud K. et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A 2001;98:13255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schumacher TN, Schreiber RD.. Neoantigens in cancer immunotherapy. Science 2015;348:69–74. [DOI] [PubMed] [Google Scholar]
- 32. Chen DS, Mellman I.. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 33. Llosa NJ, Cruise M, Tam A. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le DT, Durham JN, Smith KN. et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. André T, Overman M, Lonardi S. et al. Analysis of tumor PD-L1 expression and biomarkers in relation to clinical activity in patients (pts) with deficient DNA mismatch repair (dMMR)/high microsatellite instability (MSI-H) metastatic colorectal cancer (mCRC) treated with nivolumab (NIVO) + ipilimumab (IPI): CheckMate 142 [ESMO abstract 484PD]. Ann Oncol 2017;28(Suppl 5):I63. [Google Scholar]
- 36. Chalabi M, Fanchi LF, Van den Berg JG. et al. Neoadjuvant ipilimumab plus nivolumab in early stage colon cancer [ESMO abstract LBA37_PR]. Ann Oncol 2018;29:mdy424. [Google Scholar]
- 37. Ciardiello D, Vitiello PP, Cardone C. et al. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat Rev 2019;76:22–32. [DOI] [PubMed] [Google Scholar]
- 38. Popat S, Hubner R, Houlston RS.. Systematic review of microsatellite instability and colorectal cancer prognosis. JCO 2005;23:609–18. [DOI] [PubMed] [Google Scholar]
- 39. Colle R, Cohen R, Cochereau D. et al. Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer 2017;104:42–51. [DOI] [PubMed] [Google Scholar]
- 40. Chen EX, Derek J, Jonker JM. et al. CCTG CO.26: updated analysis and impact of plasma-detected microsatellite stability (MSS) and tumor mutation burden (TMB) in a phase II trial of durvalumab (D) plus tremelimumab (T) and best supportive care (BSC) versus BSC alone in patients (pts) with refractory metastatic colorectal carcinoma (rmCRC) [ASCO abstract]. J Clin Oncol 2019;37:3512. [Google Scholar]
- 41. Baretti M, Le DT.. DNA mismatch repair in cancer. Pharmacol Ther 2018;189:45–62. [DOI] [PubMed] [Google Scholar]
- 42. Gandini S, Massi D, Mandala M.. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2016;100:88–98. [DOI] [PubMed] [Google Scholar]
- 43. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 44. Hersom M, Jorgensen JT.. Companion and complementary diagnostics-focus on PD-L1 expression assays for PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer. Ther Drug Monit 2018;40:9–16. [DOI] [PubMed] [Google Scholar]
- 45. Shah M, Adenis A, Enzinger P. et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase 3 KEYNOTE-181 study [ASCO abstract 4010]. J Clin Oncol 2019;37:4010. [Google Scholar]
- 46. Tabernero J, Van Cutsem E, Bang Y-J. et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study [ASCO abstract LBA4007-LBA07]. JCO 2019;37:LBA4007. [Google Scholar]
- 47. Patel SP, Kurzrock R.. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847–56. [DOI] [PubMed] [Google Scholar]
- 48. Aguiar PN Jr, Santoro IL, Tadokoro H. et al. The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: a network meta-analysis. Immunotherapy 2016;8:479–88. [DOI] [PubMed] [Google Scholar]
- 49. Wang X, Teng F, Kong L. et al. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luchini C, Bibeau F, Ligtenberg MJL. et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–43. [DOI] [PubMed] [Google Scholar]
- 51. Yang L, Xue R, Pan C.. Prognostic and clinicopathological value of PD-L1 in colorectal cancer: a systematic review and meta-analysis. Onco Targets Ther 2019;12:3671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gros A, Robbins PF, Yao X. et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014;124:2246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thommen DS, Koelzer VH, Herzig P. et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018;24:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Llosa NJ, Luber B, Tam AJ. et al. Intratumoral adaptive immunosuppression and type 17 immunity in mismatch repair proficient colorectal tumors. Clin Cancer Res 2019;25:5250. 10.1158/1078-0432.ccr-19-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. AlDubayan SH, Giannakis M, Moore ND. et al. Inherited DNA-repair defects in colorectal cancer. Am J Hum Genet 2018;102:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pearlman R, Frankel WL, Swanson B. et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol 2017;3:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lorans M, Dow E, Macrae FA. et al. Update on hereditary colorectal cancer: improving the clinical utility of multigene panel testing. Clin Colorectal Cancer 2018;17:e293–305. [DOI] [PubMed] [Google Scholar]
- 58. Arana ME, Kunkel TA.. Mutator phenotypes due to DNA replication infidelity. Semin Cancer Biol 2010;20:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chalmers ZR, Connelly CF, Fabrizio D. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dunn GP, Old LJ, Schreiber RD.. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329–60. [DOI] [PubMed] [Google Scholar]
- 61. Kim R, Emi M, Tanabe K.. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007;121:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stewart TJ, Abrams SI.. How tumours escape mass destruction. Oncogene 2008;27:5894–903. [DOI] [PubMed] [Google Scholar]
- 63. Schrock AB, Ouyang C, Sandhu J. et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol 2019;30:1096–103. [DOI] [PubMed] [Google Scholar]
- 64. Georgiadis A, Durham JN, Keefer LA. et al. Noninvasive detection of microsatellite instability and high tumor mutation burden in cancer patients treated with PD-1 blockade. Clin Cancer Res 2019; 10.1158/1078-0432.CCR-19-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guinney J, Dienstmann R, Wang X. et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yaeger R, Chatila WK, Lipsyc MD. et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018;33:125–36.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Domingo E, Freeman-Mills L, Rayner E. et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol Hepatol 2016;1:207–16. [DOI] [PubMed] [Google Scholar]
- 69. Gong J, Wang C, Lee PP. et al. Response to PD-1 blockade in microsatellite stable metastatic colorectal cancer harboring a POLE mutation. J Natl Compr Canc Netw 2017;15:142–7. [DOI] [PubMed] [Google Scholar]
- 70. Li B, Li T, Pignon JC. et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet 2016;48:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McGranahan N, Furness AJ, Rosenthal R. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Anagnostou V, Smith KN, Forde PM. et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 2017;7:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Samstein RM, Lee CH, Shoushtari AN. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chan TA, Yarchoan M, Jaffee E. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Galon J, Costes A, Sanchez-Cabo F. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- 76. Mlecnik B, Bindea G, Angell HK. et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016;44:698–711. [DOI] [PubMed] [Google Scholar]
- 77. Pages F, Mlecnik B, Marliot F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391:2128–39. [DOI] [PubMed] [Google Scholar]
- 78. Galon J, Mlecnik B, Bindea G. et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014;232:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun G, Dong X, Tang X. et al. The prognostic value of immunoscore in patients with colorectal cancer: a systematic review and meta-analysis. Cancer Med 2019;8:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Van den Eynde M, Mlecnik B, Bindea G. et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 2018;34:1012–26.e3. [DOI] [PubMed] [Google Scholar]
- 81. Mlecnik B, Van den Eynde M, Bindea G. et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst 2018;110:97–108. [DOI] [PubMed] [Google Scholar]
- 82. Angelova M, Mlecnik B, Vasaturo A. et al. Evolution of metastases in space and time under immune selection. Cell 2018;175:751–65.e16. [DOI] [PubMed] [Google Scholar]
- 83. Carrasco J, Gizzi M, Pairet G. et al. Pathological responses after angiogenesis or EGFR inhibitors in metastatic colorectal cancer depend on the chemotherapy backbone. Br J Cancer 2015;113:1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chakrabarti S, Huebner LJ, Finnes HD. et al. Intratumoral CD3+ and CD8+ T-cell densities in patients with DNA mismatch repair–deficient metastatic colorectal cancer receiving programmed cell death-1 blockade. JCO Precis Oncol 2019;37:3532. [DOI] [PubMed] [Google Scholar]
- 85. Yoon HH, Shi Q, Heying EN. et al. Intertumoral heterogeneity of CD3(+) and CD8(+) T-cell densities in the microenvironment of DNA mismatch-repair-deficient colon cancers: implications for prognosis. Clin Cancer Res 2019;25:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fontana E, Eason K, Cervantes A. et al. Context matters—consensus molecular subtypes of colorectal cancer as biomarkers for clinical trials. Ann Oncol 2019;30:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lenz HJ, Ou FS, Venook AP. et al. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (alliance). JCO 2019;37:1876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aderka D, Stintzing S, Heinemann V.. Explaining the unexplainable: discrepancies in results from the CALGB/SWOG 80405 and FIRE-3 studies. Lancet Oncol 2019;20:e274–83. [DOI] [PubMed] [Google Scholar]
- 89. Becht E, de Reynies A, Giraldo NA. et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res 2016;22:4057–66. [DOI] [PubMed] [Google Scholar]
- 90. Angelova M, Charoentong P, Hackl H. et al. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol 2015;16:64.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pitroda SP, Khodarev NN, Huang L. et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun 2018;9:1793.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsantoulis P, Hill LA, Walker SM. et al. Association of a specific innate immune response to DNA damage with DNA repair deficient colorectal cancers [ASCO abstract 3035]. J Clin Oncol 2016. [Google Scholar]
- 93. Viaud S, Saccheri F, Mignot G. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Iida N, Dzutsev A, Stewart CA. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Routy B, Le Chatelier E, Derosa L. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 96. Matson V, Fessler J, Bao R. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gopalakrishnan V, Spencer CN, Nezi L. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vetizou M, Pitt JM, Daillere R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chau I. Clinical development of PD-1/PD-L1 immunotherapy for gastrointestinal cancers: facts and hopes. Clin Cancer Res 2017;23:6002–11. [DOI] [PubMed] [Google Scholar]
- 100. Tesniere A, Apetoh L, Ghiringhelli F. et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 2008;20:504–11. [DOI] [PubMed] [Google Scholar]
- 101. Antonia SJ, Villegas A, Daniel D. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 102. Yi M, Qin S, Zhao W. et al. The role of neoantigen in immune checkpoint blockade therapy. Exp Hematol Oncol 2018;7:28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Formenti SC, Demaria S.. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012;84:879–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Whiteside TL, Demaria S, Rodriguez-Ruiz ME. et al. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res 2016;22:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Twyman-Saint Victor C, Rech AJ, Maity A. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Duffy AG, Makarova-Rusher OV, Pratt D. et al. A pilot study of AMP-224, a PD-L2 Fc fusion protein, in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer [ASCO abstract 560]. J Clin Oncol 2016;34:560. [Google Scholar]
- 107. Segal NH, Kemeny NE, Cercek A. et al. Non-randomized phase II study to assess the efficacy of pembrolizumab (Pem) plus radiotherapy (RT) or ablation in mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) patients [ASCO abstract 3539]. J Clin Oncol 2016;34:3539. [Google Scholar]
- 108. Parikh AR, Clark JW, Yon-Li Wo J.. A phase II study of ipilimumab and nivolumab with radiation in microsatellite stable (MSS) metastatic colorectal adenocarcinoma (mCRC) [ASCO abstract 3514]. J Clin Oncol 2019;37:3515. [Google Scholar]
- 109. Hurwitz H, Fehrenbacher L, Novotny W. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- 110. Tabernero J, Yoshino T, Cohn AL. et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499–508. [DOI] [PubMed] [Google Scholar]
- 111. Van Cutsem E, Tabernero J, Lakomy R. et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. JCO 2012;30:3499–506. [DOI] [PubMed] [Google Scholar]
- 112. Douillard JY, Oliner KS, Siena S. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. [DOI] [PubMed] [Google Scholar]
- 113. Van Cutsem E, Lenz HJ, Kohne CH. et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. JCO 2015;33:692–700. [DOI] [PubMed] [Google Scholar]
- 114. Kroemer G, Galluzzi L, Kepp O. et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 115. Krysko DV, Garg AD, Kaczmarek A. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012;12:860–75. [DOI] [PubMed] [Google Scholar]
- 116. Galluzzi L, Buque A, Kepp O. et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97–111. [DOI] [PubMed] [Google Scholar]
- 117. Vincent J, Mignot G, Chalmin F. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70:3052–61. [DOI] [PubMed] [Google Scholar]
- 118. Tanis E, Julie C, Emile JF. et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur J Cancer 2015;51:2708–17. [DOI] [PubMed] [Google Scholar]
- 119. Carmeliet P, Jain RK.. Angiogenesis in cancer and other diseases. Nature 2000;407:249–57. [DOI] [PubMed] [Google Scholar]
- 120. Chen DS, Hurwitz H.. Combinations of bevacizumab with cancer immunotherapy. Cancer J 2018;24:193–204. [DOI] [PubMed] [Google Scholar]
- 121. Hegde PS, Wallin JJ, Mancao C.. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2018;52:117–24. [DOI] [PubMed] [Google Scholar]
- 122. Villadangos JA, Schnorrer P.. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol 2007;7:543–55. [DOI] [PubMed] [Google Scholar]
- 123. Oyama T, Ran S, Ishida T. et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 1998;160:1224–32. [PubMed] [Google Scholar]
- 124. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58–62. [DOI] [PubMed] [Google Scholar]
- 125. Motz GT, Santoro SP, Wang LP. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014;20:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ohm JE, Gabrilovich DI, Sempowski GD. et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 2003;101:4878–86. [DOI] [PubMed] [Google Scholar]
- 127. Voron T, Colussi O, Marcheteau E. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015;212:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vanneman M, Dranoff G.. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bendell JC, Powderly JD, Lieu CH. et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC) [ASCO abstract 704]. J Clin Oncol 2015;33. [Google Scholar]
- 130. Wallin J, Pishvaian MJ, Hernandez G. et al. Clinical activity and immune correlates from a phase Ib study evaluating atezolizumab (anti-PDL1) in combination with FOLFOX and bevacizumab (anti-VEGF) in metastatic colorectal carcinoma [AACR abstract 2651]. Cancer Res 2016;76:2651. [Google Scholar]
- 131. Pozzi C, Cuomo A, Spadoni I. et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med 2016;22:624–31. [DOI] [PubMed] [Google Scholar]
- 132. Dechant M, Weisner W, Berger S. et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res 2008;68:4998–5003. [DOI] [PubMed] [Google Scholar]
- 133. Inoue Y, Hazama S, Suzuki N. et al. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci 2017;108:455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stein A, Binder M, Al-Batran S-E. et al. Avelumab and cetuximab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (MCRC): results of the safety run-in phase of the phase II AVETUX trial (AIO-KRK-0216) [ASCO abstract]. J Clin Oncol 2018;36:3561. [Google Scholar]
- 135. Ebert PJR, Cheung J, Yang Y. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 2016;44:609–21. [DOI] [PubMed] [Google Scholar]
- 136. Liu L, Mayes PA, Eastman S. et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res 2015;21:1639–51. [DOI] [PubMed] [Google Scholar]
- 137. Brea EJ, Oh CY, Manchado E. et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol Res 2016;4:936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Eng C, Kim TW, Bendell J. et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019;20:849–61. [DOI] [PubMed] [Google Scholar]
- 139. Fruman DA, Rommel C.. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xue G, Zippelius A, Wicki A. et al. Integrated Akt/PKB signaling in immunomodulation and its potential role in cancer immunotherapy. J Natl Cancer Inst 2015. [DOI] [PubMed] [Google Scholar]
- 141. Lee S, Margolin K.. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3:3856–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Steel JC, Waldmann TA, Morris JC.. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012;33:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Robinson TO, Schluns KS.. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol Lett 2017;190:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zboralski D, Hoehlig K, Eulberg D. et al. Increasing tumor-infiltrating T cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 blockade. Cancer Immunol Res 2017;5:950–6. [DOI] [PubMed] [Google Scholar]
- 145. Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med 2016;48:e242.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. van de Laar L, Coffer PJ, Woltman AM.. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 2012;119:3383–93. [DOI] [PubMed] [Google Scholar]
- 147. Mach N, Gillessen S, Wilson SB. et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res 2000;60:3239–46. [PubMed] [Google Scholar]
- 148. Qin Z, Noffz G, Mohaupt M. et al. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J Immunol 1997;159:770–6. [PubMed] [Google Scholar]
- 149. Gillessen S, Naumov YN, Nieuwenhuis EE. et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion. Proc Natl Acad Sci U S A 2003;100:8874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hennequart M, Pilotte L, Cane S. et al. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res 2017;5:695–709. [DOI] [PubMed] [Google Scholar]
- 151. Takikawa O, Yoshida R, Kido R. et al. Tryptophan degradation in mice initiated by indoleamine 2, 3-dioxygenase. J Biol Chem 1986;261:3648–53. [PubMed] [Google Scholar]
- 152. McGaha TL, Huang L, Lemos H. et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev 2012;249:135–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. van Baren N, Van den Eynde BJ.. Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol Res 2015;3:978–85. [DOI] [PubMed] [Google Scholar]
- 154. Ferdinande L, Decaestecker C, Verset L. et al. Clinicopathological significance of indoleamine 2, 3-dioxygenase 1 expression in colorectal cancer. Br J Cancer 2012;106:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Afzal MZ, Mercado RR, Shirai K.. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer 2018;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Palmer TM, Trevethick MA.. Suppression of inflammatory and immune responses by the A(2A) adenosine receptor: an introduction. Br J Pharmacol 2008;153(Suppl 1):S27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zarek PE, Huang CT, Lutz ER. et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008;111:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Schuler PJ, Saze Z, Hong CS. et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol 2014;177:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Segal NH, Saro J, Melero I. et al. Phase I studies of the novel carcinoembryonic antigen T-cell bispecific (CEA-CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients (pts) with metastatic colorectal cancer (mCRC) [ESMO abstract 403P]. Ann Oncol 2017;28(Suppl 5):v134. [Google Scholar]
- 160. Gershovich PM, Karabelskii AV, Ulitin AB. et al. The role of checkpoint inhibitors and cytokines in adoptive cell-based cancer immunotherapy with genetically modified T cells. Biochemistry (Moscow) 2019;84:695–710. [DOI] [PubMed] [Google Scholar]