Figure 3.
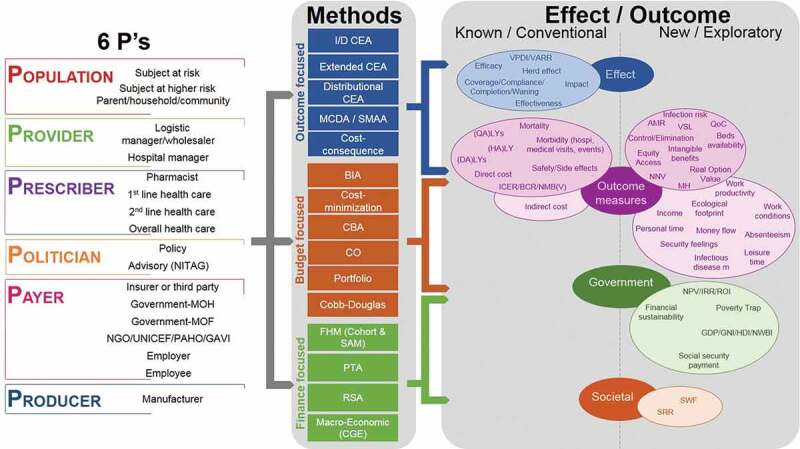
Making the links between the stakeholder types (Figure 2), the effect/outcome measured (Table 1) and the analysis methods (Table 2)
AMR, antimicrobial resistance; BCR, benefit–cost ratio; BIA, budget impact analysis; CBA, cost-benefit analysis; CEA, cost-effectiveness analysis; CGE, computable general equilibrium models; CO, constrained optimisation; DALY, disability-adjusted life-year; FHM, fiscal health modelling; GAVI, Global Alliance for Vaccines and Immunization; GDP, gross domestic product; GNI, gross national income; HALY, health-adjusted life-year; HDI, human development index; I/DCEA, incremental/decremental CEA; I/DCER, incremental/decremental cost-effectiveness ratio; IRR, internal rate of return; LY, life-year; MCDA, multi-criteria decision-making analysis; MOF, Ministry of Finance; MOH, Ministry of Health; NGO, non-governmental organisation; NITAG, national immunisation technical advisory group; NMB(V), net monetary benefit (value); NNV, number needed to vaccinate; NPV, net present value; NWBI, national well-being index; PAHO, Pan-American Health Organization; PTA, poverty trap avoidance; QALY, quality-adjusted life-year; QoC, quality of care; ROI, return on investment; RSA, risk-sharing agreements; SMAA, stochastic multi-criteria acceptability analysis; SRR, social rate of return; SWF, social welfare function; UNICEF, United Nations Children’s Fund; VARR, vaccine-attributable rate reduction; VPDI, vaccine-preventable disease incidence; VSL, value of a statistical life.
