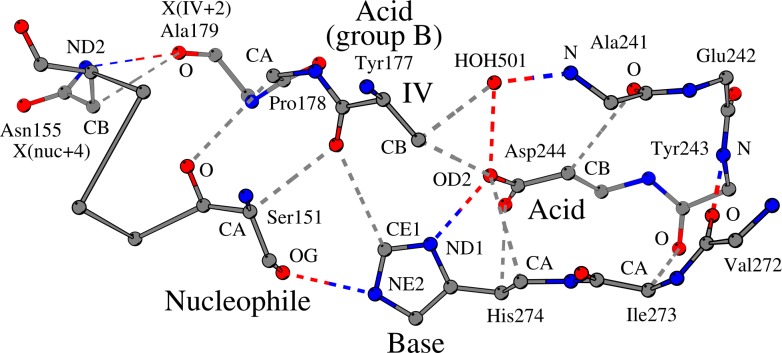
Fig 2. Coordination of the catalytic nucleophile-histidine pair and the catalytic histidine-acid pair in the carboxylesterase SshEstI (PDB ID:3WJ1).
The catalytic nucleophile (“Nucleophile”, Ser151 in SshEstI) is hydrogen bonded (OG/Ser151 –NE2/His274) to the catalytic histidine (“Base”, His274 in SshEstI) as part of the standard interaction network of the residues of the catalytic machinery. However, three additional conserved interactions ensure the fine turning of the two catalytic residues next to each other: The main-chain oxygen atom of Tyr177 that is located at the C-terminus of strand β6 (termed and shown as “IV”) forms two weak hydrogen bonds: one with the catalytic nucleophile (O/Tyr177 –CA/Ser151) and another with the catalytic histidine (O/Tyr177 –CE1/His274); a third contact (O/Ser151 –CA/Pro178) is formed between the catalytic nucleophile and Pro178 (located ahead of position IV) and coordinates the catalytic nucleophile. Thus, these three interactions support the optimal arrangement of the catalytic nucleophile-histidine pair. The catalytic histidine (His274) interacts (ND1/His274 –OD2/Asp244) with the catalytic acid (“Acid”, Asp244 in SshEstI) and is further supported by two weak hydrogen bonds: OD2/Asp244 –CA/His274 and OD1/Asp244 –CB/His274. Interactions of the catalytic acid zone that are associated with the coordination of the catalytic histidine and other contacts located nearby the catalytic site are shown. Gray dashed lines, weak hydrogen bonds; colored dashed lines, standard hydrogen bonds.