Anti-HIV-1 activity and cytotoxicity of the novel phenylalanine derivatives II-10(a–o) and III-15(a–o).
| ||||
|---|---|---|---|---|
| Compounds | R | EC50a (μM) | CC50b (μM) | SIc |
| II-10a |
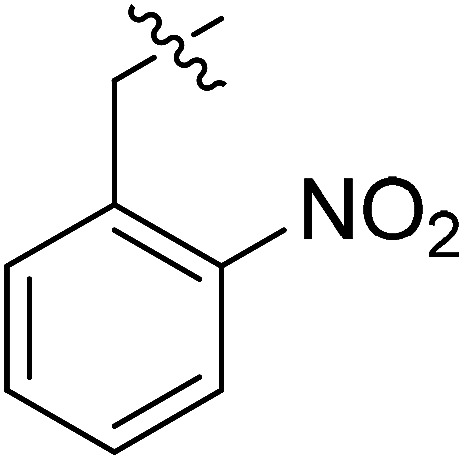
|
>35.49 | >35.49 | NDe |
| II-10b |
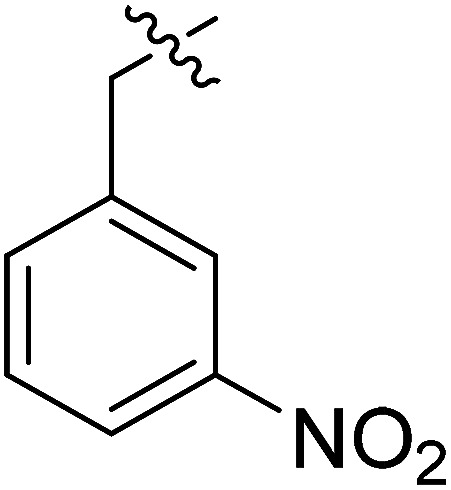
|
>35.49 | >35.49 | NDe |
| II-10c |
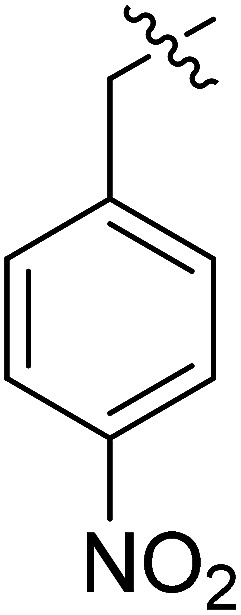
|
2.13 ± 0.75 | >35.49 | >16.66 |
| II-10d |
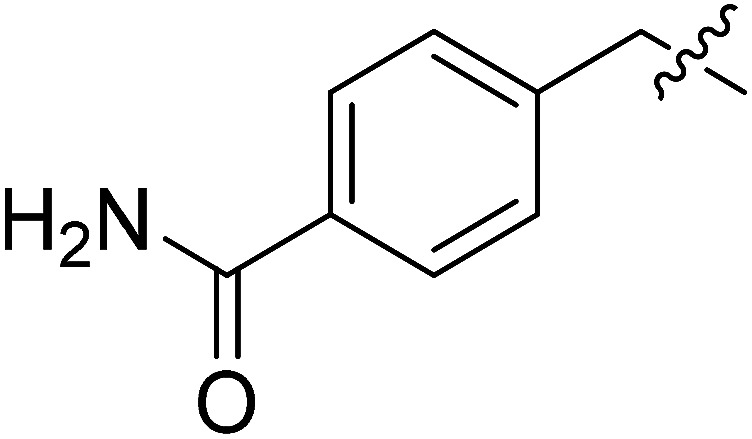
|
23.37 ± 6.88 | >32.02 | >1.37 |
| II-10e |
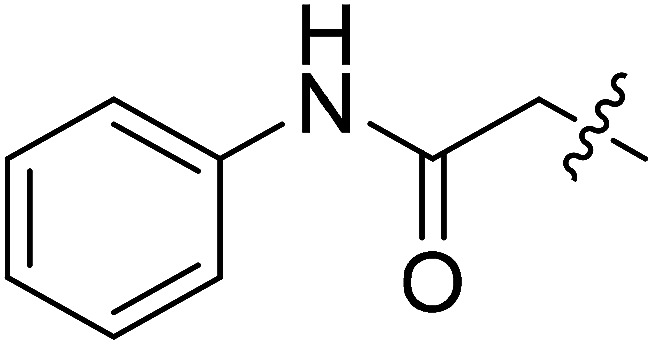
|
8.16 ± 2.56 | >32.01 | >3.92 |
| II-10f |
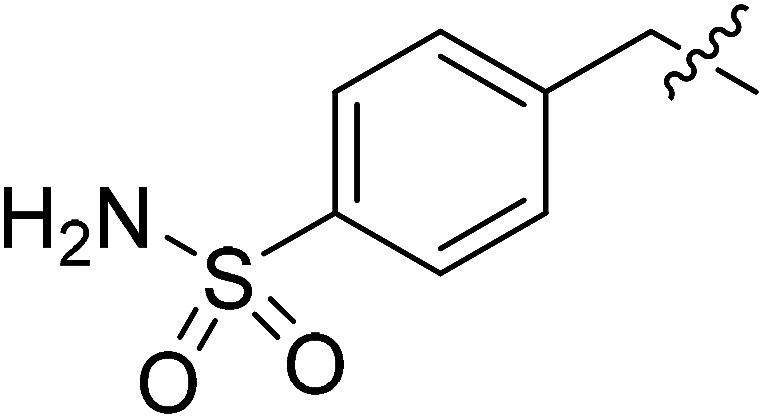
|
>30.27 | >30.27 | NDe |
| II-10g |
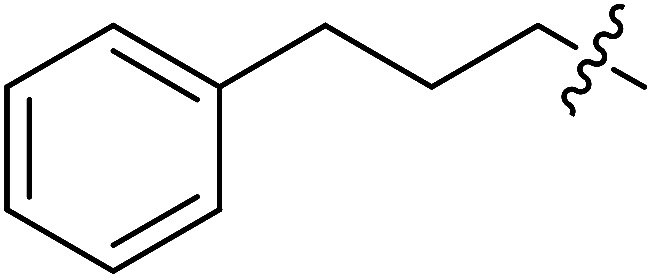
|
>32.80 | >32.80 | NDe |
| II-10h |
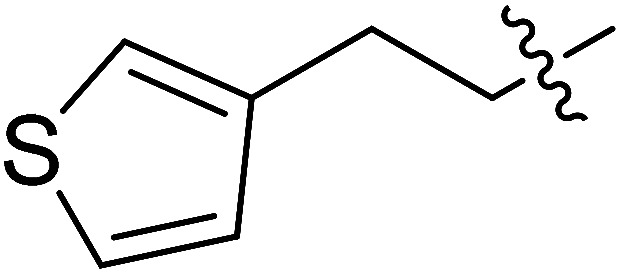
|
7.48 ± 2.83 | >33.24 | >4.44 |
| II-10i |
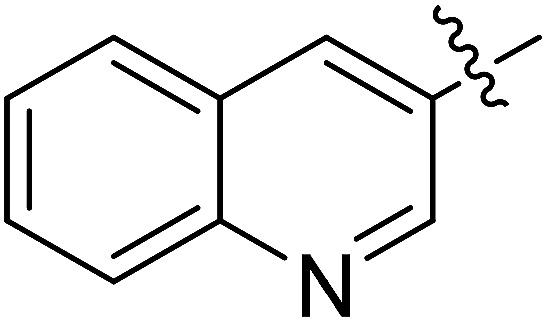
|
21.50 ± 4.53 | >32.32 | >1.50 |
| II-10j |
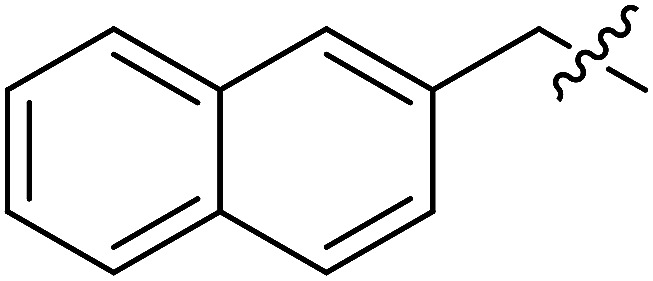
|
>31.66 | >31.66 | NDe |
| II-10k |
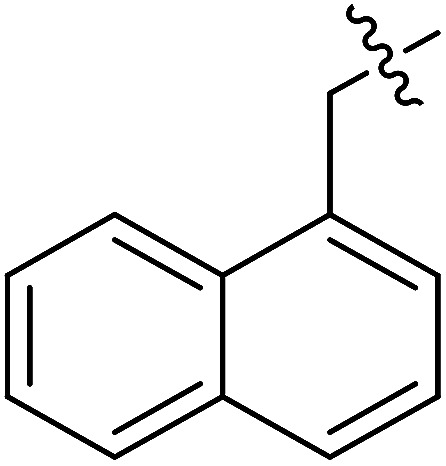
|
>31.66 | >31.66 | NDe |
| II-10l | CH3OC OCH2– | NAd | NAd | NDe |
| II-10m | CH3CH2OC OCH2– | NAd | NAd | NDe |
| II-10n | CH3OC O(CH2)3– | >33.80 | >33.80 | NDe |
| II-10o |
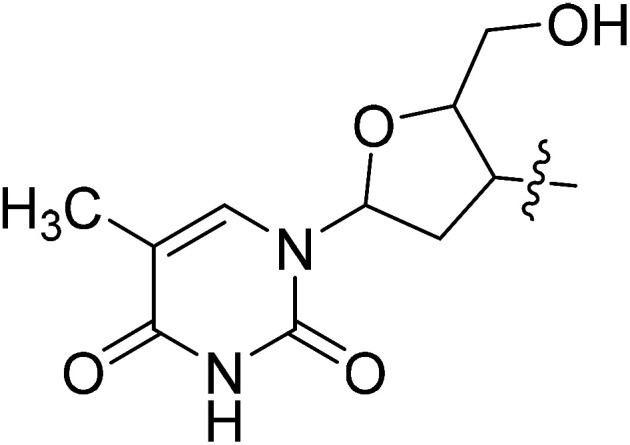
|
>27.94 | >27.94 | NDe |
| III-15a |
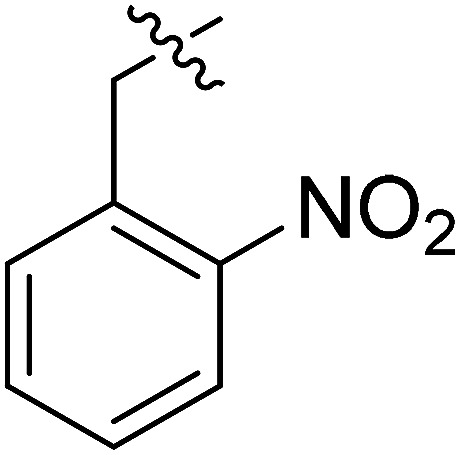
|
>33.86 | >33.86 | NDe |
| III-15b |
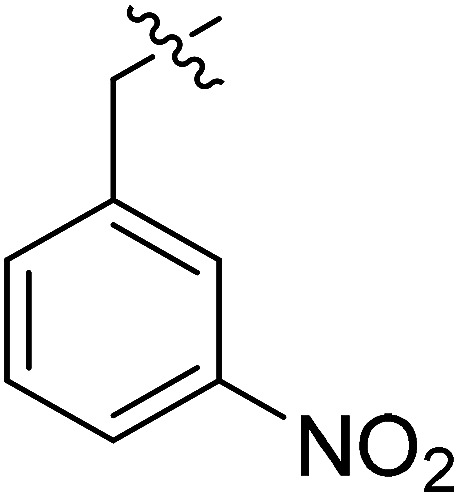
|
>33.86 | >33.86 | NDe |
| III-15c |
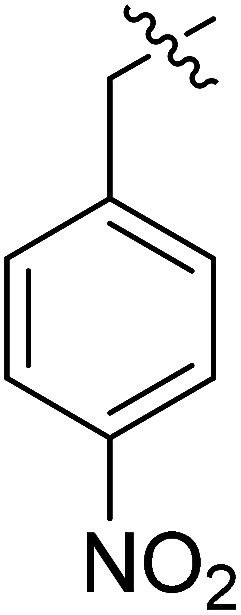
|
>33.86 | >33.86 | NDe |
| III-15d |
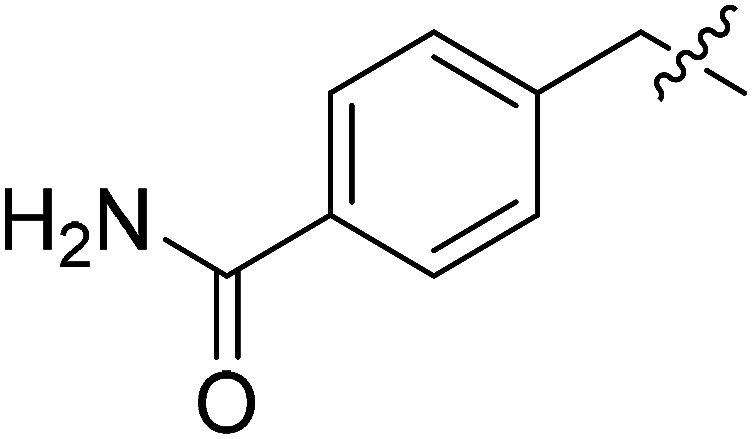
|
26.16 ± 7.30 | >33.97 | >1.30 |
| III-15e |
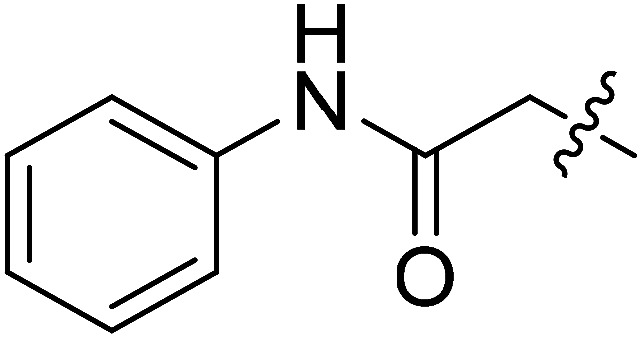
|
>33.97 | >33.97 | NDe |
| III-15f |
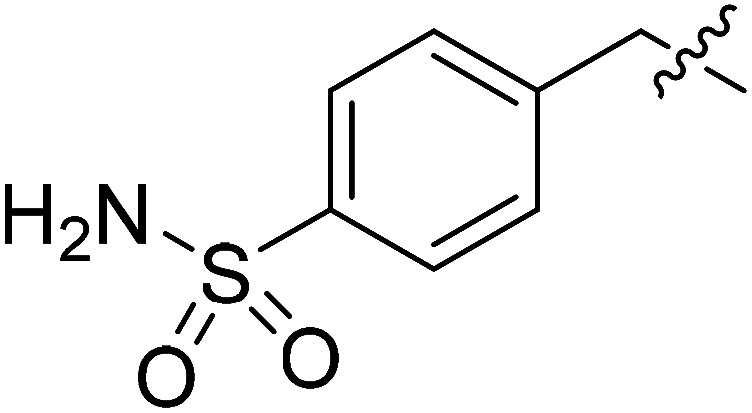
|
>32.01 | >32.01 | NDe |
| III-15g |
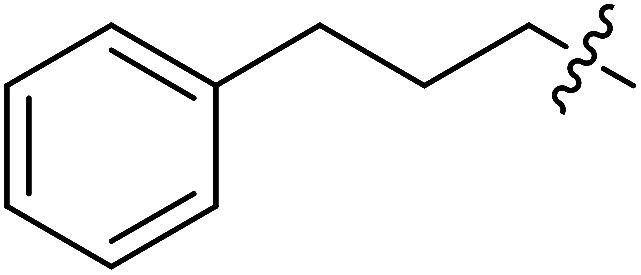
|
>34.86 | >34.86 | NDe |
| III-15h |
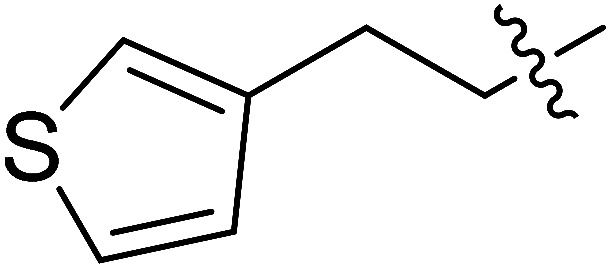
|
9.37 ± 3.18 | >35.36 | >3.77 |
| III-15i |
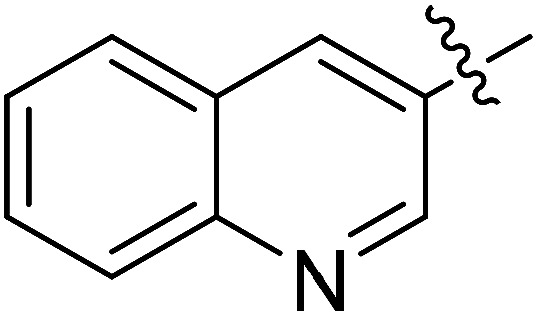
|
>34.33 | >34.33 | NDe |
| III-15j |
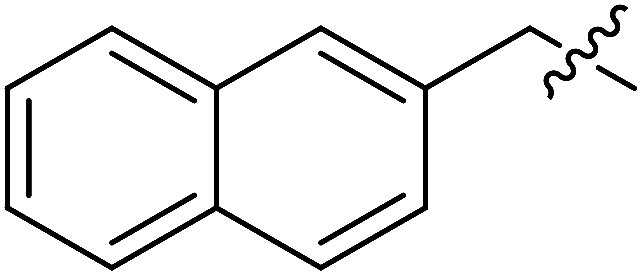
|
>33.57 | >33.57 | NDe |
| III-15k |
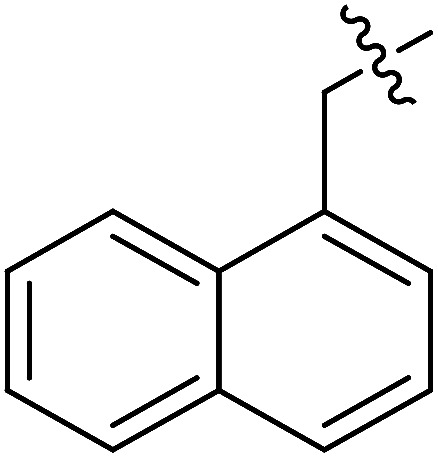
|
>33.57 | >33.57 | NDe |
| III-15l | CH3OC OCH2– | NAd | NAd | NDe |
| III-15m | CH3CH2OC OCH2– | NAd | NAd | NDe |
| III-15n | CH3OC O(CH2)3– | >35.99 | >35.99 | NDe |
| III-15o |
|
>29.42 | >29.42 | NDe |
| PF-74 | — | 0.52 ± 0.18 | >47.00 | >90.38 |
EC50: the concentration of the compound required to achieve 50% protection of TZM-bl cells against HIV-1-induced cytopathic effect, as determined by the MTT method.
CC50: the concentration of the compound required to reduce the viability of uninfected cells by 50%, as determined by the MTT method.
SI: selectivity index, the ratio of CC50/EC50.
NA: no anti-HIV-1 activity or cytotoxicity at the test concentration.
ND: not determined.
