Fig. 6. Phosphomimetic substitutions at the PEST domain and charged crowding demonstrate a general electrostatic basis of IDR/ETS interactions.
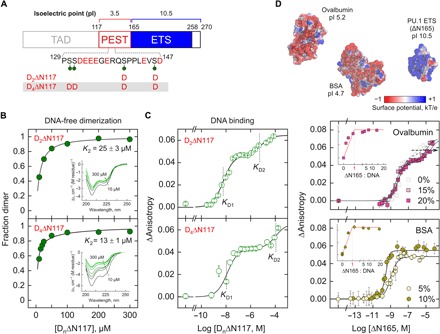
(A) Schematic of the phosphorylated Ser residues in human PU.1, marked by green pins. The Ser→Asp substituted positions in D2ΔN117 and D4ΔN117 are shown. (B) D2ΔN117 and D4ΔN117 exhibit enhanced dimeric propensities without DNA relative to ΔN117 (compare to Fig. 3D). (C) D2ΔN117 and D4ΔN117 are progressively impaired in 2:1 DNA complex formation. (D) The anionic crowders ovalbumin and BSA modulate DNA binding by ΔN117 in a similar way as the phosphomimetic substitutions on ΔN117 to an extent that correlates with their sizes and low pIs. Inset: Stoichiometric determination using 1 μM DNA (first binding transition in the case of ovalbumin). The spacing of the ordinates is identical to main plots. The surface potentials of the structures were computed using the Adaptive Poisson-Boltzmann Solver (APBS) at 0.15 NaCl.
