Figure 3. BMX ablation augments thrombin-mediated permeability in mice and in cultured ECs.
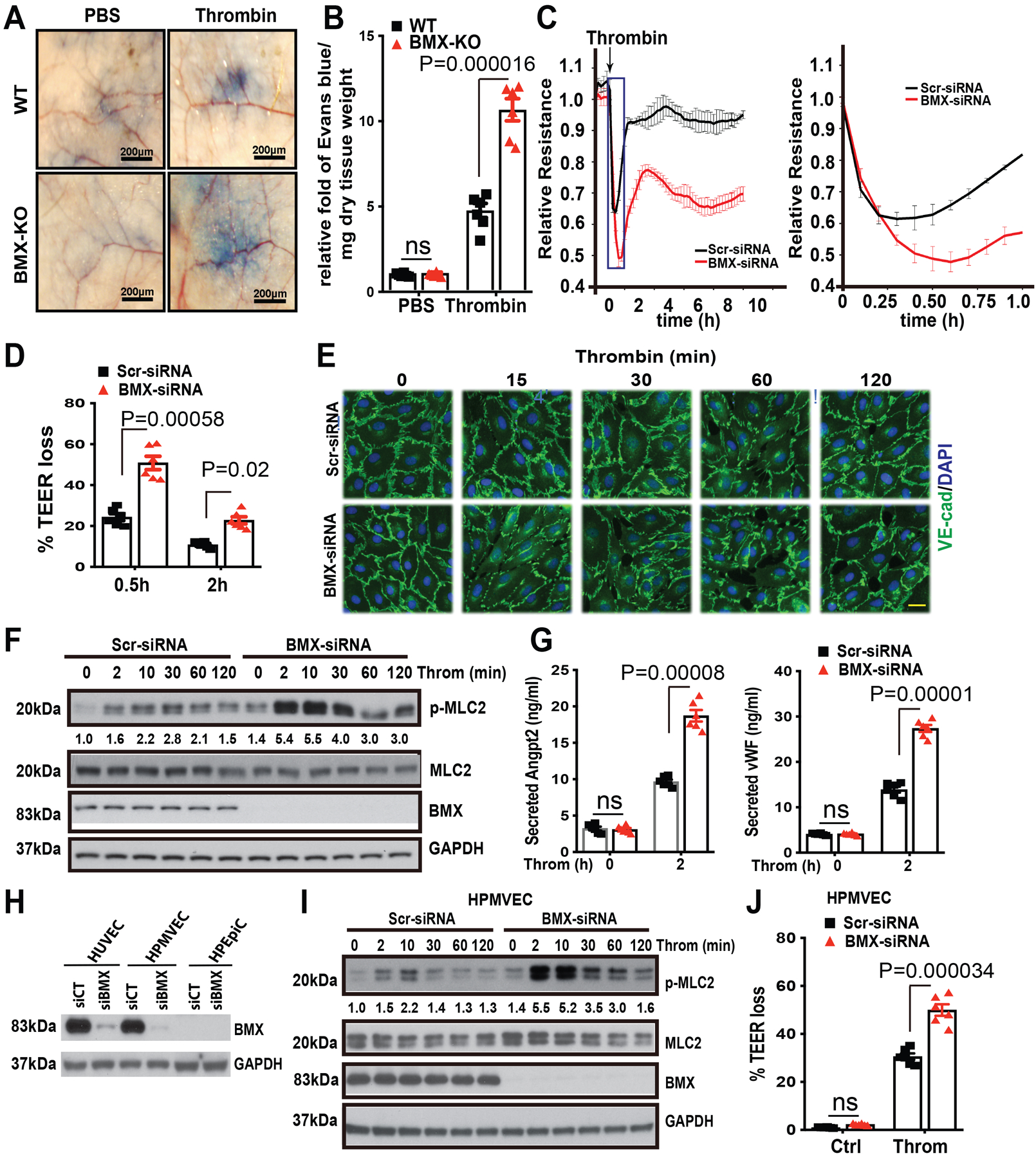
(A) The mouse tail vein was injected with Evans blue dye (30 mg/ml), and then thrombin (20 ng/ml) or phosphate-buffered saline was immediately injected into the ventral skin. (B) The relative fold of Evans blue dye per mg of dry tissue weight was quantified, n=6. (C) Post-confluent HUVECs were treated with thrombin (1.5 U/ml) as shown by the arrow, and then TEER was immediately measured for the indicated times. An expanded 0–1 h window is shown on the right. (D) % TEER losses at indicated times are presented. (E-F) HUVECs were transfected with Scr-siRNA or BMX-siRNA. About 72 h after transfection, HUVECs were starved overnight and then were treated with thrombin (1.5 U/ml) at the indicated times, followed by immunofluorescence analysis of VE-Cadherin (E) or by Western blot for thrombin signaling as indicated (F). (G) After starvation overnight, Scr-siRNA-transfected or BMX-deficient HUVECs were treated with thrombin (1.5 U/ml) for 2 h, and then the medium was collected for ELISA to test secreted Ang2 and vWF levels. (H) HUVECs, HPMVECs and HPEpiC were transfected with Scr-siRNA or BMX-siRNA. BMX and PAR1 protein levels were determined by Western blot with specific antibodies. (I) HPMVECs were transfected with Scr-siRNA or BMX-siRNA. About 72 h after transfection, HPMVECs were starved overnight and then were treated with thrombin (1.5 U/ml) at the indicated times, followed by Western blot for thrombin signaling as indicated. (J) Post-confluent HPMVECs were treated with thrombin (1.5 U/ml) and then TEER was measured. % TEER losses at 0.5 h are presented. All experiments were repeated three times with biological replicates. Error bars indicate SEM. ns: non significance; P < 0.05 were considered to indicate statistical significance between WT and BMX-KO mice (B) or between Scr-siRNA and BMX-siRNA groups (D,G,J) using two-way ANOVA and Bonferroni post-hoc multiple comparisons. Scale bar: 200 μm (A); 20 μm (E).
