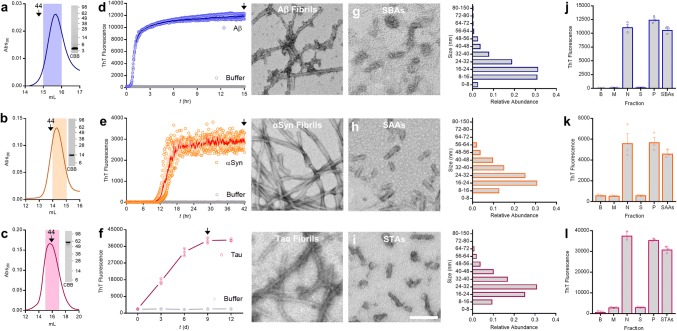
Fig. 1.
Preparation and characterization of soluble aggregates of Aβ, α-synuclein and tau. a–c Representative chromatograms depicting isolation of monomeric (a) Aβ1–42, (b) α-synuclein (αSyn), and (c) tau. Downward arrows indicate elution of globular molecular weight standards, the shaded region indicates the fraction retained for aggregation experiments and the inset SDS-PAGE/coomassie (CBB) depicts migration of the purified monomers. d–f Freshly SEC-isolated monomer was diluted to 20 µM (Aβ1-42; d) and 35 µM (αSyn; e) and 50 µM (tau; f), combined with Thioflavin-T (ThT) and aggregation monitored until ThT signals plateaued (Tmax). Aβ and αSyn were aggregated without additives while tau was aggregated in the presence of 50 and 100 µM heparin and DTT, respectively. Binding of ThT to Aβ1–42 and αSyn was monitored continuously, whereas aliquots of tau were removed, mixed with ThT and assessed at 3-day intervals. In each case, identical reactions without protein (buffer) served as negative control. Fibrils were harvested at time points indicated by downward arrows, mounted on grids and representative negative-stain electron microscope (EM) micrographs of Aβ, αSyn and tau Tmax fibrils are presented to the right of each graph. g–i Representative negative-stain EM micrographs of immersion sonicated soluble aggregates (Aβ SBAs, g; αSyn SAAs, h; tau STAs, i). Size distribution of SBAs, SAAs and STAs as determined by negative-stain EM are presented to the right of each micrograph. j–l Neat Tmax fibrils (N), the supernatant of centrifuged fibrils (S), resuspended washed fibril pellets (P), and SBAs (j), SAAs (k), and STAs (l) were used for ThT binding. Buffer alone (B) and freshly isolated monomer (M) were included as negative controls. Scale bar in micrographs from d–i = 100 nm. Data in d–f and j–l are the mean ± SD, indicate three technical replicates and are representative of at least three independent experiments. Molecular weight markers (in kDa) are indicated to the right of the inset CBB gels in a, b and c