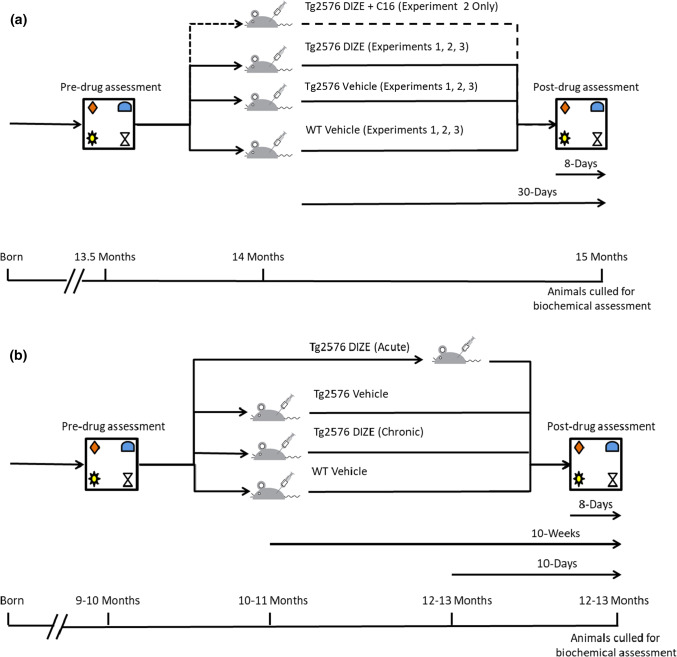
Fig. 1.
Schematic representation of the experimental design in a for Experiments 1, 2 and 3 and b for Experiment 4. a Male mice between 13 and 14 months of age were subject to pre-drug assessment in the object-in-place (OiP)-associative recognition memory task to establish a baseline measurement. Mice were then randomly assigned into groups to ensure that there was no significant difference in behaviour prior to treatment. All WT mice were administered vehicle (saline) only. In Experiment 1, Tg2576 mice were administered either vehicle (saline) or DIZE (15 mg/kg/day) for 30 days. In Experiment 2, a third Tg2576 group was added that was administered DIZE + C16 (25 mg/kg/day) for 30 days. In Experiment 3, both WT and Tg2576 groups were administered vehicle or DIZE. In each experiment, on day 23 of treatment, post-drug assessment commenced with habituation and testing was performed on days 28 and 30. All mice were culled and brains were dissected on day 30 immediately following post-testing and underwent pathological and biochemical assessment. b Mice underwent pre-drug assessment at 9–10 months of age and all mice were found to be asymptomatic. The mice were then divided into four groups: WT vehicle, Tg2576 vehicle, Tg2576 DIZE (chronic) and Tg2576 DIZE (acute). Tg2576 groups were allocated based on OiP performance so that all groups showed comparable levels of contact time and DR score performance. All groups except Tg2576 DIZE acute commenced IP DIZE treatment (15 mg/kg/day) or vehicle for 10 weeks. At 8 days prior to the final two test days at 12–13 months of age, DIZE acute mice received drug treatment daily for 10 days (15 mg/kg/day). Tg2576 mice had previously been shown to be cognitively impaired at 12–13 months of age in Experiments 1 and 2. Mice across all four groups were re-tested at 12–13 months of age. At the end of the study, after all mice had undergone OiP testing, they were culled immediately and underwent pathological and biochemical assessments