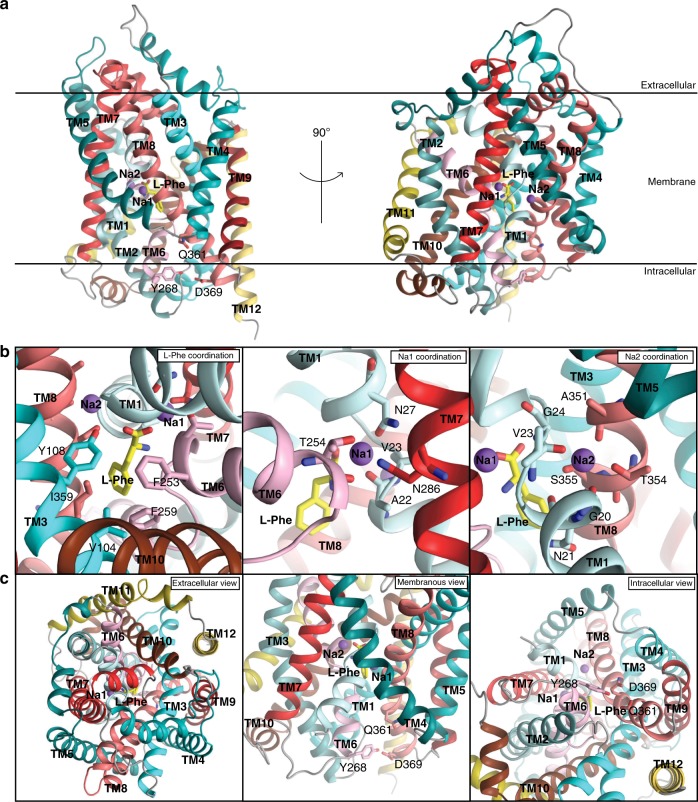
Fig. 4. Crystal structure of Na+/l-Phe-bound LeuTW8A.
a Side views of the LeuTW8A monomer seen along the plane of the lipid bilayer with two sodium ions (purple spheres) and a single l-Phe molecule (yellow sticks) bound centrally. Membrane orientation is indicated. The solved LeuTW8A structure revealed an inward-facing occluded conformation. b Close-up view of the Na+/L-Phe binding sites visualizing the residues coordinating binding of L-Phe (left), Na1 (middle) and Na2 (right). L-Phe binds similarly to l-Leu in the outward-facing occluded structure10. The space for the additional atoms in the aromatic ring of l-Phe is provided by the hydrophobic side chains lining the binding pocket. F259 forms a π-π stacking with the aromatic ring of the bound L-Phe substrate and F253 on TM6. V104 and I359 are also part of the hydrophobic pocket containing the hydrophobic side chain of the L-Phe substrate while the conserved Y108 on TM3 forms a hydrogen bond to the carboxylic acid of the bound substrate. Na1 displays octahedral coordination by A22, N27, T254, and N286 residues, and interacts directly with l-Phe. Na2 is trigonal bi-pyramidal, and established by interaction with G20, V23, A351, T354, and S355 residues (labeled and shown as colored sticks). c Close-up views of the extracellular vestibule region presented from the extracellular side (left), the membranous side (middle) and the intracellular vestibule region seen from the intracellular side (right). Internal gate residues (Y268, Q361, and D369) are labelled and shown as colored sticks. Helices are colored following the same scheme as in the schematic illustration of LeuT in Fig. 2.