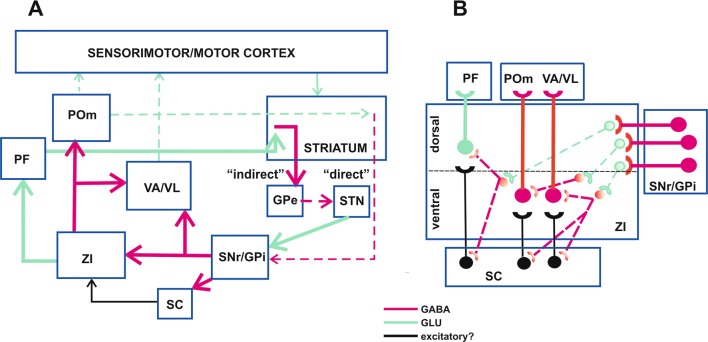
Fig. 4.
a The zona incerta (ZI) as a link in a neuronal chain responsible for conveying impulses potentially involved in motor signs of Parkinson’s disease. Due to dopaminergic deficiency in PD and imbalance between the “indirect” and “direct” GABAergic pathways arising from the striatum, the basal ganglia GABAergic output projections from the SNr/GPi to the VA/VL thalamic nuclei are overactive which results in inhibition of thalamic neurons and their projections to the sensorimotor/motor cortex [13]. The ZI receives GABAergic input from the SNr/GPi [71, 79, 87, 91] which is probably overactive, as well. The ZI, in turn, sends glutamatergic efferents to the PF [68, 77, 92] and GABAergic ones to the POm and VA/VL [68, 76, 77, 82] which are under excitatory influence of pathways originating from the SC [77], which also receives the GABAergic input from the SNr/GPi [15, 86]. Since glutamatergic pathways from the PF and POm to the striatum activate the “indirect” and “direct” pathways, respectively [77, 135, 136, 139], it has been suggested that the ZI, via inhibition of the POm, may suppress the “direct” pathway, while, via activation of the PF, may sensitize the “indirect” pathway to excitatory input from the sensorimotor/motor cortex [77, 136]. It may be speculated, therefore, that if both these incerto-thalamic pathways are overactive in PD they may contribute to the imbalance between the “direct” and “indirect” pathways and in this way, may be involved in appearance of motor signs of this disease. Moreover, putative activation of the GABAergic incerto-thalamic projection, which terminates in the VA/VL may constitute an additional, to the basal ganglia, source of inhibition of these nuclei in PD. b A hypothesis of potential, internal mechanisms of the ZI involved in modulation of incoming signals from the basal ganglia (SNr/GPi) and their transmission to thalamic nuclei (PF, POm, VA/VL) in PD. The ZI possesses extensive internal incerto-incertal network: GABAergic interneurons [107], collaterals of GABAergic incertal projection neurons [76, 99], and incerto-incertal connections linking different ZI regions utilizing undefined neurotransmitters [68, 108]. GABAergic incertal neurons are localized mainly in the ventral sector of this structure [66, 72, 77, 99], whereas glutamatergic ones concentrate mainly in its dorsal subdivision [66, 71, 72]. The GABAergic nigro(pallido)-incertal pathway (arising from the SNr/GPi) terminates mainly in the dorsal sector of the ZI [71, 79, 91], where it may switch to and inhibit glutamatergic neurons sending their axons to the ventral sector. A decrease in glutamatergic transmission in the ventral ZI may lead to inhibition of some GABAergic neurons terminating on incerto-thalamic neurons in the dorsal and ventral ZI, projecting to the PF, and POm/VA/VL, respectively [68, 76, 77, 92]. Inhibition of the internal GABAergic network may, in turn, lead to disinhibition of incerto-thalamic pathways, which are additionally under excitatory influence of signals coming from the SC [77]. Therefore, due to the above complex incerto-incertal interactions, overactivation of inhibitory basal ganglia input to the ZI, may increase output of this structure to the thalamus. Additionally, GABAergic incerto-collicular pathway starting in the ventral ZI [78–84] may be inhibited indirectly by the nigro(pallido)-incertal pathway that may result in disinhibition of the colliculo-incertal pathway and potentiation of its excitatory influence on incertal outputs to the thalamus. Brain structures: GPe external segment of the globus pallidus, GPi internal segment of the globus pallidus, PF parafascicular nucleus, POm posteromedial thalamic nucleus, SC superior colliculus, SNr substantia nigra, pars reticulata, STN subthalamic nucleus, VA/VL ventroanterior/ventrolateral nuclei of the thalamus, ZI zona incerta. Neuronal pathways: GABA GABAergic pathways, GLU glutamatergic pathways, excitatory?—putative excitatory pathways. Bold neurons and arrows—indicate activated neurons, pale neurons and dashed arrows—indicate inhibited neurons