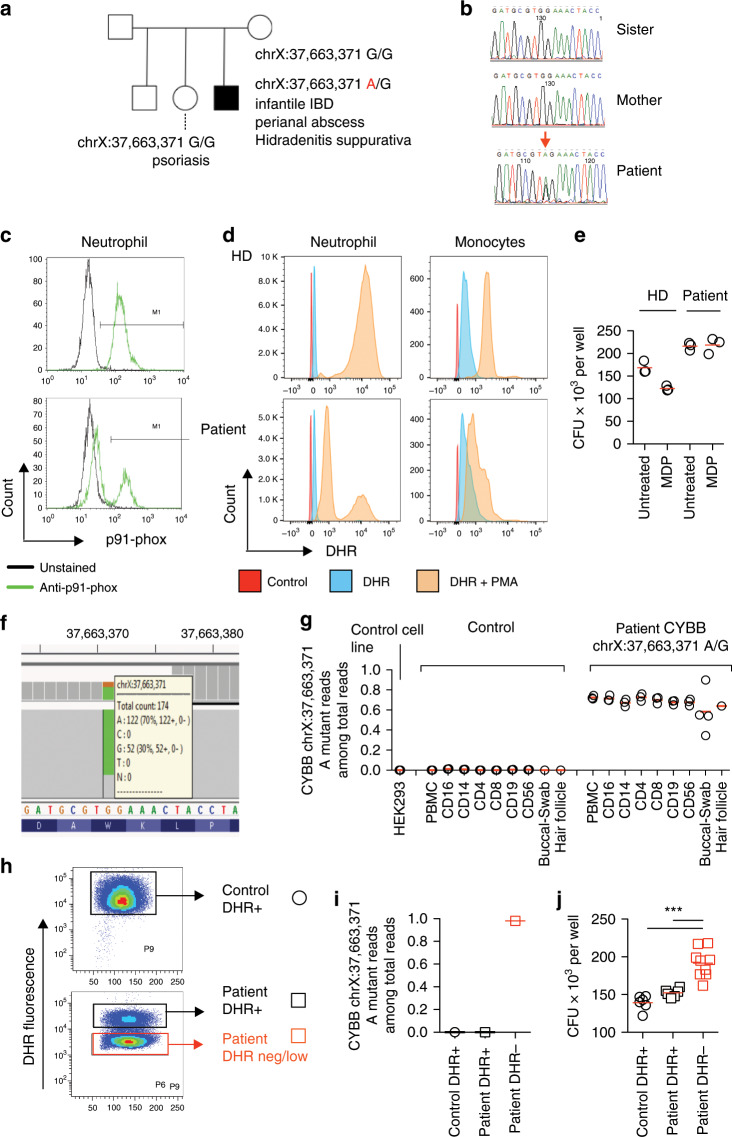
Fig. 1. Analysis of CYBB mosaicism in a male patient.
a Pedigree structure for the family of the male patient with the mosaic hemizygous mutation in CYBB (chrX:37,663,371A/G; p.W380X). b Sanger sequencing of the chrX:37,663,371 CYBB mutation site in the patient and unaffected relatives (sister and mother). c p91-phox protein expression (the gene product encoded by CYBB) analysed by flow cytometry assay (FACS). Control is a healthy donor. d Measurement of oxidative burst in neutrophils and monocytes using the dihydrorhodamine-1,2,3 (DHR) assay. Obtained from the patient and a healthy donor (control). e Defective bacterial handling in monocyte derived macrophages with the CYBB mosaicism. Intracellular survival of Salmonella typhimurium was quantified using the agar plate technique. Results show three technical replicates. Obtained from the patient and a healthy donor (control). f, g Quantification of mutant read proportion at chrX:37,663,371 using the IGV browser. PBMCs were sorted into immune cell subsets (Supplementary Figs. 6B,C) and compared with buccal swabs and hair follicles, as well as with healthy donor immune cells and a HEK293T cell line as technical control. h FACS sorting strategy for DHR-high and DHR-low populations following DHR staining and PMA stimulation (Supplementary Fig. 6A). i Quantification of mutant reads at chrX:37,663,371 following sorting based on DHR for control DHR-high, patient DHR-high, and patient DHR-low neutrophils (Supplementary Fig. 6A). j Gentamicin protection assay on neutrophils for control DHR-high, patient DHR-high, and patient DHR-low populations (Supplementary Fig. 6A). Briefly, neutrophils were infected at a MOI 1:10 for 45 min with Salmonella enterica serovar typhimurium and subsequently treated with gentamicin for 45 min. Neutrophils were then lysed and plated on LB agar plates for CFU counting on the following day. ***p < 0.001, Mann–Whitney U-test.