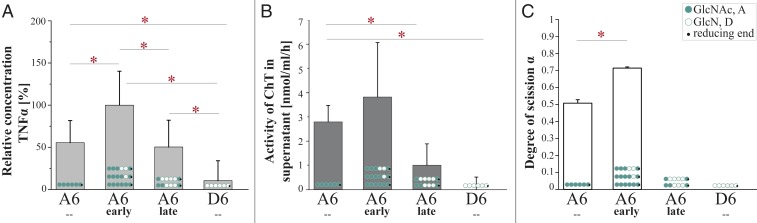
Fig. 4.
The effect of early and late CnCda4 products and A6/D6 controls on the secretion of TNFα by macrophages and the cleavage products generated by recombinant human chitotriosidase (ChT). (A) Activation of human peripheral blood-derived macrophages (HPBMs) by different chitin and chitosan hexamers (1 µg/mL). Secretion of TNFα into the supernatant was measured 6 h after stimulation. Data from five independent experiments with different batches of macrophages were normalized to the negative control (starvation medium, 0%) and to the positive control (10 ng/mL LPS, 100%). Data are presented as means ± SD (B) Activity of the human ChT measured in the supernatant after stimulation with the different chitin and chitosan hexamers (1 µg/mL). The concentration of ChT in 5 µL supernatant was determined by adding 20 mM of the ChT-specific substrate 4-methylumbelliferyl-β-d-N,N′-diacetylchitobioside hydrate in 100 mL McIlvain buffer (100 mM citric acid, 200 mM sodium phosphate, pH 5.2). The enzyme reaction was stopped after 20 min at 37 °C by adding 200 mL of 300 mM glycine-NaOH buffer, pH 10.5. Converted substrate was measured by fluorimetry at 450 nm and related to a standard of recombinant ChT. Values are means of three independent experiments ±SD. (C) Relative ChT activity on the oligomeric substrates GlcNAc6 (A6), GlcN6 (D6), and the early and late hexameric products of CnCda4. Isolated ChT (50 ng) was incubated with the oligomeric substrates (2.5 µg) for 24 h at 37 °C in 50 mM ammonium acetate (pH 4.5). Products were detected and quantified by HPLC-MS analysis, and ChT activity was quantified by the amount of hydrolyzed substrate, described as the degree of scission (α), where 100% equals complete degradation of the substrate. All data are based on five independent experiments (n = 5) and are given as means ± SD. Statistical analysis was performed using the t test (*P < 0.5).