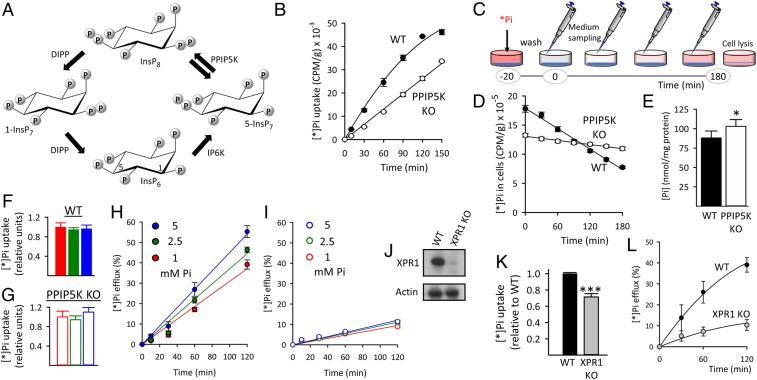
Fig. 1.
Reduced XPR1-mediated Pi efflux from HCT116 cells after either PPIP5K KO or XPR1 KO. (A) The proposed (17) cyclical pathway of PP-InsP turnover involving IP6K, PPIP5Ks (denoted by twin arrows because the enzyme has separate 1-kinase and 1-phosphatase activities), and diphosphoinositol polyphosphate phosphatases (DIPP). Carbons in InsP6 are labeled at positions 1 and 5. (B) Net [*]Pi uptake by WT and PPIP5K KO HCT116 cells. (C) Graphic depicting the timeline of the [*]Pi efflux assays. The [*]Pi efflux data are normalized (except in D) to total cell [*]Pi at zero time determined after cell lysis. (D) [*]Pi efflux from WT and PPIP5K KO HCT116 cells plotted as decreasing amounts of cell [*]Pi; CPM, counts per minute. (E) Total Pi in WT and PPIP5K HCT116 KO cells. (F and G) [*]Pi influx into WT and PPIP5K HCT116 KO cells, respectively, incubated with extracellular Pi at 1 mM (red), 2.5 mM (green), or 5 mM (blue). (H and I) [*]Pi influx into WT and PPIP5K HCT116 KO cells, respectively, incubated with extracellular Pi at 1 mM (red), 2.5 mM (green), or 5 mM (blue). (J) Western analysis of XPR1 expression in WT and XPR1 KO HCT116 cells. (K) WT and XPR1 KO HCT116 cells were labeled with [*]Pi for 20 min, and then, total [*]Pi was determined. (L) [*]Pi efflux from WT and XPR1 KO HCT116 cells prelabeled as in K. Statistical significance is indicated as follows. *P < 0.05; ***P < 0.001.