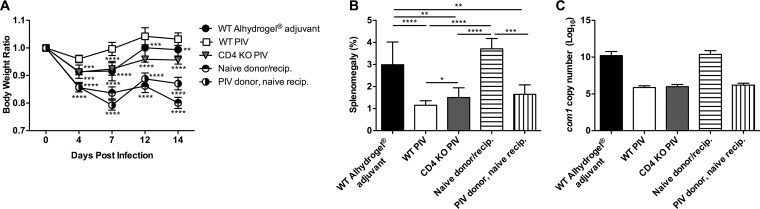
FIG 5.
CD4+ T cells from PIV-vaccinated mice are sufficient to generate protection. CD4 KO and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. Naive CD4 KO mice received 5 × 106 purified CD4+ T cells by i.p. injection from either naive or PIV-vaccinated WT mice 24 h before infection. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A) The relative body weight was measured throughout the infection. Splenomegaly (B) and splenic bacterial burden (C) were evaluated at 14 dpi to compare protection. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. Bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. The experiments with results shown in Fig. 5 and 6 were performed concurrently, so the WT Alhydrogel adjuvant, WT PIV, and CD4 KO PIV groups represented in these figures are identical. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Dunnett’s multiple-comparison test [A] or one-way ANOVA with Tukey’s multiple-comparison test [B and C]).