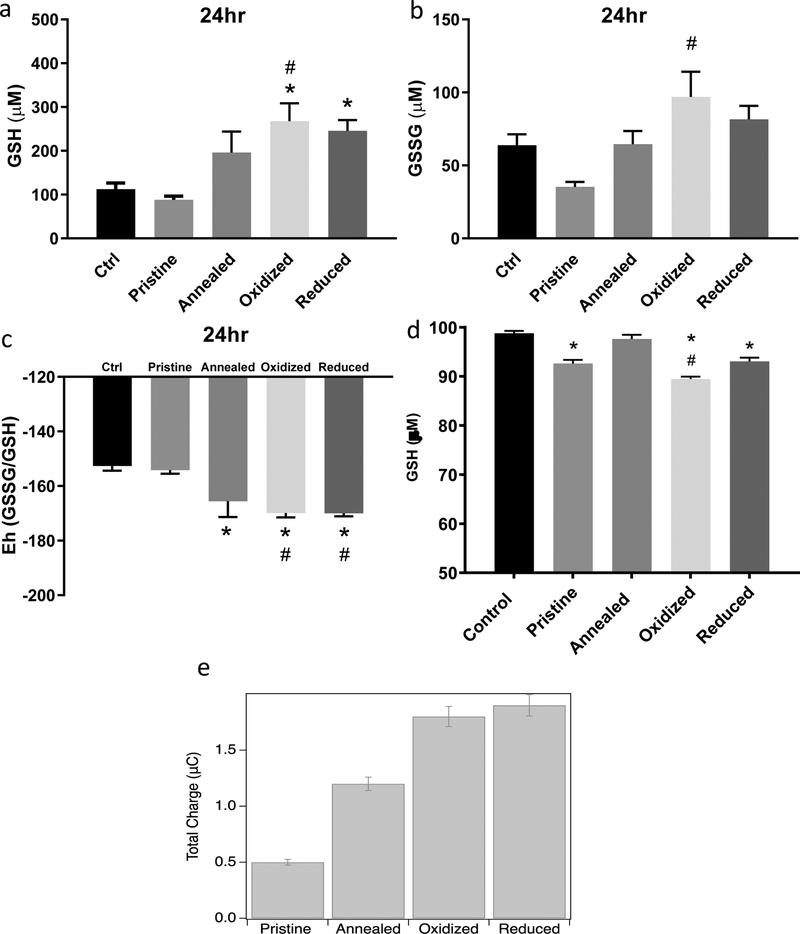
Figure 6: Glutathione Redox Potential Measurement in Rat Aortic Endothelial Cells Exposed to ZnO Nanoparticles and Charge Transfer.
RAECs were exposed to pristine, annealed, oxidized or reduced ZnO NPs for 24hrs at 20 μg/ml. Intracellular reduced and oxidized glutathione (GSH (A) and GSSG (B) respectively) concentrations were measured using HPLC (N=3). (C) The redox potential (Eh) was calculated using the Nernst equation (Eh=E0 + (RT/nF)ln([acceptor]/[donor]). (D) Measurement of ZnO NPs direct reactivity with cell free GSH incubated for 15 min at 37 °C as measured by Elman’s reagent. (E) ZnO NP cyclic voltammetry for the measurement of charge transfer between gluthathione and ZnO NPs. A three-electrode setup measured the charge transfer of ZnO (1 mg/ml) nanoparticles with and without chemical defects to gluthathione (1mM). *statistically significant compared to control (untreated cells). #statistically significant compared to pristine ZnO NPs (p<0.05).