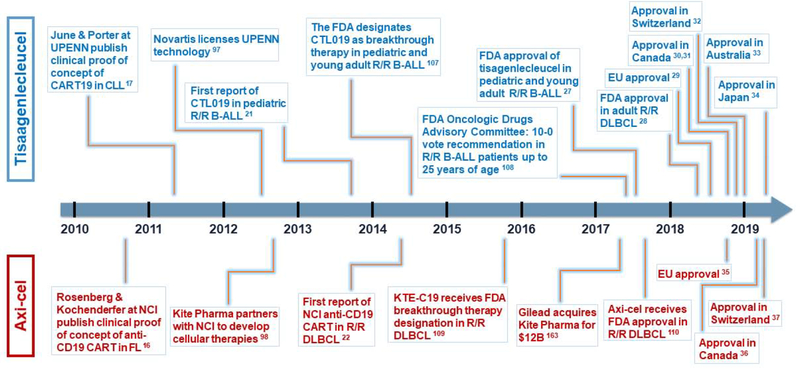
Figure 1. Similar trajectories led to FDA approval of first two gene-edited cellular therapies for cancer.
Above timeline (blue): landmarks of tisagenlecleucel road to approval. Below timeline (red): landmarks leading to axicabtagene ciloleucel approval. UPENN, University of Pennsylvania. CART, chimeric antigen receptor T cell. CLL, chronic lymphocytic leukemia. FL, follicular lymphoma. NCI, National Cancer Institute. B-ALL, B-cell acute lymphoblastic leukemia. DLBCL, diffuse large B cell lymphoma. FDA, US Food and Drug Administration. R/R, relapsed-refractory. EU, European Union. Axi-cel, axicabtagene ciloleucel.