Abstract
Objective:
To estimate whether maternal sense of control in labor is associated with breastfeeding at 4–8 weeks postpartum.
Methods:
This is a secondary analysis of data from a multicenter randomized controlled trial of elective induction of labor at 39 weeks gestation in low-risk nulliparous women. In this trial, women completed the Labor Agentry Scale, a validated measure of women’s feelings of control over the childbirth process, 6–96 hours after delivery. The LAS score, which is higher with more perceived control during childbirth, was analyzed both as a continuous and a categorical variable (quintiles). Self-reported breastfeeding at 4–8 weeks postpartum was categorized as exclusive breast, breast and bottle, or exclusive bottle feeding. Women were included in this analysis if they labored, filled out a LAS questionnaire, had a neonate who survived until the postpartum visit, and provided information on infant feeding. Multinomial logistic regression was used to adjust for confounders.
Results:
Of 5,185 women, 32.9% (N=1,705) were exclusively breastfeeding, 31.2% (N=1,620) were breast and bottle feeding, and 35.9% (N=1,860) were exclusively bottle feeding 4–8 weeks after delivery. Overall LAS score ranged from 34 to 203 (median 167, interquartile range (IQR) 145–182). The median LAS score was 169 (IQR 151–183) for women exclusively breastfeeding, 166 (IQR 142–182) for women who were breast and bottle feeding, and 164 (IQR 142–181) for women bottle feeding only (p < 0.001). In the unadjusted multinomial model, women with LAS scores in the lowest two quintiles (ie, those with lower perceived control during childbirth) were less likely to be exclusively breastfeeding (as compared with those exclusively bottle feeding) than women in the highest LAS quintile. When controlling for confounders, however, this association was no longer significant.
Conclusion:
After adjustment for confounders, perceived control during childbirth was not associated with breastfeeding at 4–8 weeks postpartum among nulliparous women.
Clinical Trial Registration:
Précis
Maternal perception of control during childbirth is not associated with breastfeeding at 4–8 postpartum.
Introduction:
Research on breastfeeding has focused on the short-term and long-term benefits for mothers and infants, such as postpartum weight loss1 and decreased risk of cancers such as endometrial cancer among mothers2, lower risk of childhood obesity3, and decreased risk of Sudden Infant Death Syndrome.4 Breastfeeding even has important societal benefits, such as decreased health care costs.5
Many studies have examined extrinsic factors that are associated with breastfeeding, such as maternal demographic factors6, length of maternal time off from work7, and availability of facilities and time to pump breastmilk.8 Other studies have examined objective aspects of the birth process such as mode of delivery9 and use of medications, such as oxytocin, during labor10 on breastfeeding duration. There has been less research regarding a woman’s subjective experience of the birth process, which may influence the likelihood of breastfeeding.11
The Labor Agentry Scale (LAS) is a validated measure for a woman’s sense of control over the labor process.12 Decreased feelings of control and psychosocial stress during labor, as measured by the LAS, have been associated with post-traumatic stress disorder at 6 weeks postpartum13, delayed onset of lactation14, and decreased patient satisfaction with the birth process15, all of which are associated with decreased breastfeeding rates.16–18
The objective of this analysis was to determine whether a woman’s sense of control in labor, as measured by the LAS, is associated with the likelihood of breastfeeding at 4–8 weeks postpartum. We hypothesized that higher sense of perceived control would be associated with increased likelihood of breastfeeding.17–22
Methods:
This was a secondary analysis of the ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial, a multicenter randomized controlled trial of elective induction of labor at 39 weeks of gestation versus expectant management in low-risk nulliparous women. Full details of recruitment, screening, eligibility criteria, enrollment, and the sample size of the ARRIVE trial are published elsewhere.19
As part of the ARRIVE trial, women completed the Labor Agentry Scale, a validated measure of women’s feelings of control over the childbirth process,12 within 6–96 hours after delivery. The LAS score, which is higher with more perceived control during childbirth, was analyzed both as a continuous and categorical variable, with the total score divided into quintiles.
For our study, women were included for analysis if they underwent spontaneous labor or were induced, had a live birth, had an infant who was alive at the time of the postpartum visit, and had a postpartum visit between 4 and 8 weeks postpartum. Women who did not fill out the LAS questionnaire or who did not provide information on infant feeding were excluded. Women with known HIV positivity (a recognized contraindication to breastfeeding) were excluded from the ARRIVE trial because of modified delivery plan.
Our primary outcome was self-reported breastfeeding at the postpartum visit, defined as exclusive breastfeeding, breast and bottle feeding, or exclusive bottle feeding. For descriptive analyses, we used the chi square test for categorical variables, and the Kruskal-Wallis test or Wilcoxon rank-sum test for continuous variables. We used multinomial logistic regression to control for confounders. As a sensitivity analysis, we also used multivariable logistic regression modeling infant feeding as a dichotomous outcome: exclusive breastfeeding versus all other feeding method (exclusive bottle feeding and breast and bottle feeding), and any breastfeeding (including women who underwent breast and bottle feeding) versus exclusive bottle feeding.
We assessed several variables as potential confounders, including maternal age, maternal race and ethnicity, maternal body mass index (BMI; kg/m2) at admission to labor and delivery, marital status, primary source of payment for prenatal care, maternal employment, alcohol use at any time during the pregnancy, smoking at any time during the pregnancy, mode of delivery (spontaneous vaginal, operative vaginal, and cesarean), type of pregnancy (spontaneous vs. any artificial reproductive technology), type of labor (spontaneous, augmented, or induced), a composite of any major maternal complication (blood transfusion, hysterectomy, wound complication requiring wound to be reopened, ICU admission), maternal pain score (women were asked within 6–96 hours after delivery to rate their labor pain on a 10-point Likert scale with higher scores indicating greater pain20), birthweight, a composite outcome of perinatal death or severe neonatal complications (need for respiratory support within 72 hours after birth, Apgar score of 3 or less at 5 minutes, hypoxic-ischemic encephalopathy, seizure, confirmed sepsis, confirmed pneumonia, meconium aspiration syndrome, bone fracture, neurologic injury, retinal hemorrhage, intracranial hemorrhage, subgaleal hemorrhage, or hypotension requiring vasosupport), treatment group (induction versus expectant management), and neonatal intermediate or intensive care unit admission. The final model was a parsimonious model, with variables significant at the p < 0.05 level included.
All analyses were carried out using SAS software (SAS institute, Cary, NC). Variables were considered statistically significant at the p < 0.05 level. The ARRIVE trial was approved by the Institutional Review Boards of all participating centers (including Northwestern University) and registered at https://clinicaltrials.gov/ (). All patients gave their informed consent to participate in the ARRIVE trial.
Results:
The study population is summarized in Figure 1. Of the 6,106 women randomized in the ARRIVE trial, 6,033 (98.8%) had delivery data, labored and had a live neonate. Ten women either withdrew or were lost to follow up before delivery, 58 women did not labor, and there were 5 perinatal deaths. Of the remaining 6,033 women, 563 (9.3%) were missing information on infant feeding, 212 (3.5%) were missing information on the LAS questionnaire, and 73 (1.2%) were missing information on both. These cases with missing information were excluded leaving a sample size of 5,185 women (84.9% of total randomized cohort). The women included in the analysis, compared with those excluded due to missing data, were more likely to be white, married or living with partner, employed, had private insurance, were induced, and had a slightly lower overall pain score (Table 1).
Figure 1:
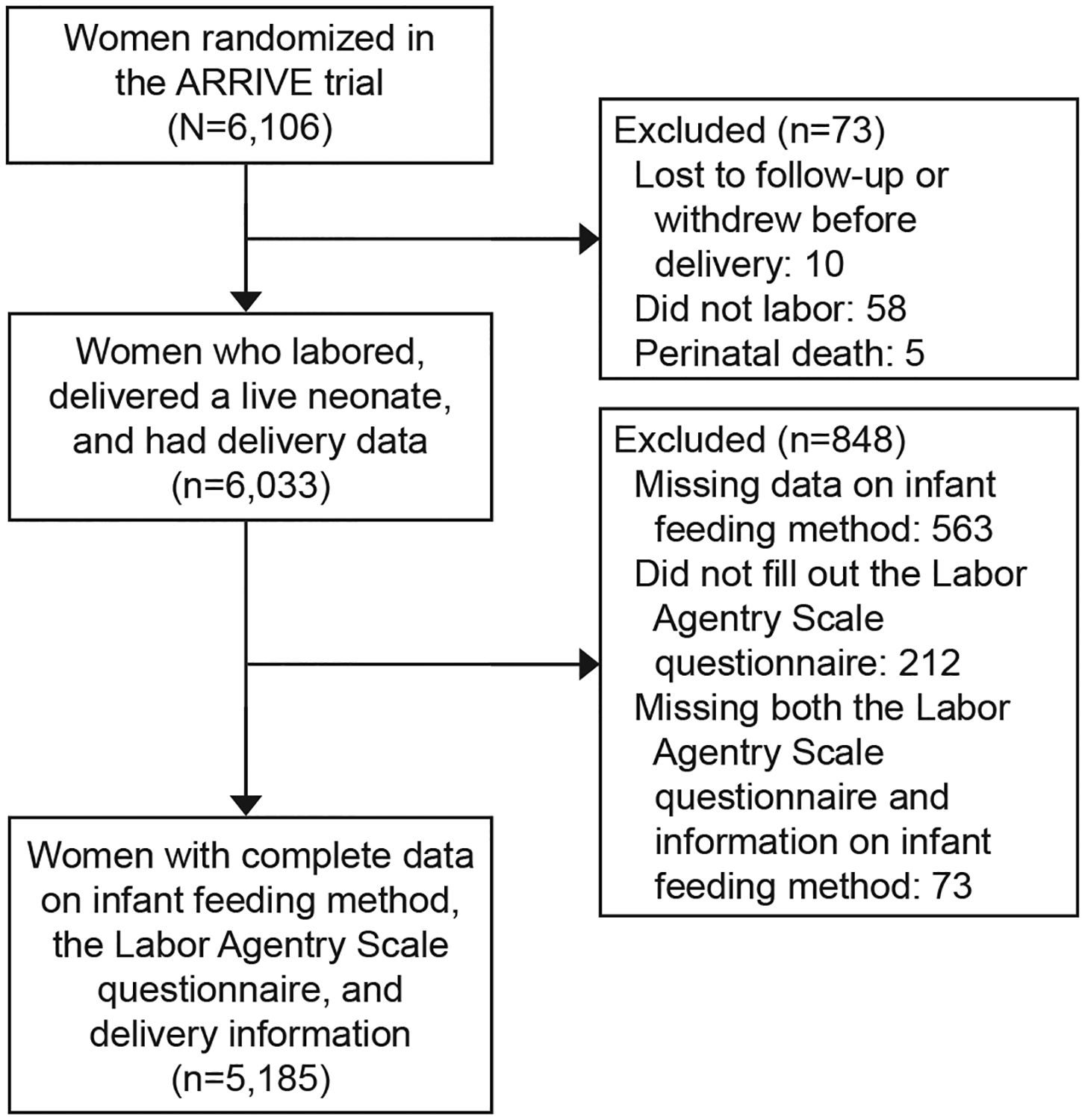
Study population. ARRIVE, A Randomized Trial of Induction Versus Expectant Management.
Table 1.
Maternal and Infant Characteristics Included and Excluded in Analysis
| Included | Excluded | P value * | |
|---|---|---|---|
| (N=5185) | (N=848) | ||
| Baseline variables | |||
| Age - yr | 24 (21–28) | 24 (20–29) | 0.90 |
| Race or ethnic group † | |||
| Non-Hispanic black | 1183 (22.8) | 213 (25.1) | <0.001 |
| Non-Hispanic white | 2376 (45.8) | 283 (33.4) | |
| Hispanic | 1369 (26.4) | 277 (32.7) | |
| Asian, other, unknown, or more than one race | 257 (5.0) | 75 (8.8) | |
| BMI at labor and delivery admission - kg/m2 ‡ | 30.6 (27.5– 35.0) | 30.4 (27.1–34.7) | 0.21 |
| Married or living with partner | 3103 (59.9) | 466 (55.0) | 0.01 |
| Employment status ‡ | <0.001 | ||
| Full-time | 2085 (40.3) | 320 (37.8) | |
| Part-time | 631 (12.2) | 56 (6.6) | |
| None | 2454 (47.5) | 470 (55.6) | |
| Private insurance ‡ | 2383 (46.0) | 327 (38.6) | <0.001 |
| Smoked cigarettes | 389 (7.5) | 70 (8.3) | 0.44 |
| Drank alcohol ‡ | 206 (4.0) | 30 (3.5) | 0.54 |
| Method of conception was ovulation induction, artificial insemination or in vitro fertilization | 131 (2.5) | 24 (2.8) | 0.60 |
| Study group assignment was induction of labor | 2641 (50.9) | 394 (46.5) | 0.02 |
| Labor & delivery variables | |||
| Type of labor | <0.001 | ||
| Spontaneous | 770 (14.9) | 158 (18.6) | |
| Spontaneous, augmented | 1305 (25.2) | 239 (28.2) | |
| Induced | 3110 (60.0) | 451 (53.2) | |
| Successful delivery type | 0.18 | ||
| Spontaneous vaginal | 3755 (72.4) | 616 (72.6) | |
| Forceps / vacuum vaginal | 424 (8.2) | 55 (6.5) | |
| Cesarean | 1006 (19.4) | 177 (20.9) | |
| Overall pain ‡ | 7 (5–8) | 7 (5–9) | <0.001 |
| Major maternal complications | 90 (1.7) | 21 (2.5) | 0.14 |
| Neonatal variables | |||
| Birthweight - grams | 3340 (3070–3610) | 3330 (3060–3577) | 0.28 |
| Neonatal intermediate or intensive care unit admission | 622 (12.0) | 119 (14.0) | 0.09 |
| Perinatal death or severe neonatal complications composite | 250 (4.8) | 38 (4.5) | 0.67 |
Abbreviations: BMI, body mass index.
Data are n (%) or median (interquartile range) unless otherwise specified.
Based on chi-square or Wilcoxon rank-sum tests.
Participant reported race or ethnicity group.
Number of missing values: BMI (28), employment status (17), private insurance (1), drank alcohol (1), and overall pain (271).
At the postpartum visit, 1,705 women (32.9%) were exclusively breastfeeding, 1,620 women (31.2%) were breast and bottle feeding, and 1,860 women (35.9%) were exclusively bottle feeding. The median LAS score for women in the exclusive breastfeeding group was 169 (IQR 151–183), whereas it was 166 for the breast and bottle feeding group (IQR 142–182) and 164 for the exclusive bottle feeding group (IQR 142–181, p < 0.001). Overall, the LAS score ranged from 34 to 203; the median was 167 and the interquartile range (IQR) was 145–182. Figure 2 shows the distribution of infant feeding method by LAS quintile.
Figure 2:
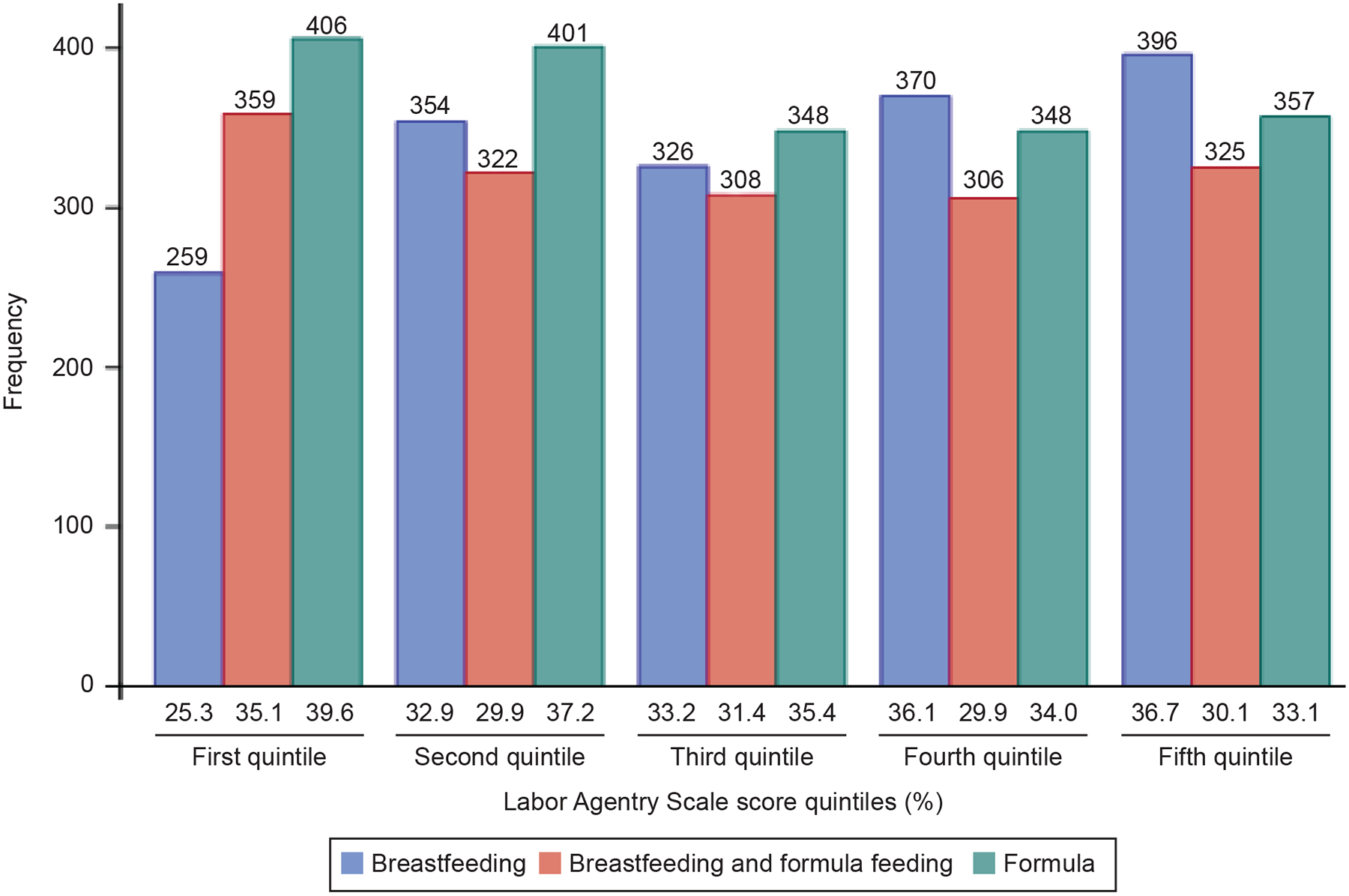
Distribution of feeding method by Labor Agentry Scale score quintile.
Table 2 shows patient characteristics by quintiles of the LAS score with higher quintiles indicating more perceived control. The women who reported more perceived control were slightly older (p= 0.01), white (p < 0.001), had slightly lower BMI (p < 0.001), were married (p < 0.001), worked fulltime (p < 0.001), had private insurance (p < 0.001), did not smoke (p < 0.02), were in the induction of labor arm (p < 0.006), had a successful spontaneous vaginal delivery (p < 0.001), reported lower pain scores (p < 0.001), and did not have major complications (p= 0.003).
Table 2.
Maternal and Infant Characteristics by LAS Score
| P value † | ||||||
|---|---|---|---|---|---|---|
| (N=1024) | (N=1077) | (N=982) | (N=1024) | (N=1078) | ||
| Baseline variables (maternal) | ||||||
| Age - yr | 23 (20–28) | 23 (20–28) | 24 (21–28) | 24 (21–28) | 24 (21–28) | 0.01 |
| Race or ethnic group ‡ | ||||||
| Non-Hispanic black | 278 (27.2) | 251 (23.3) | 228 (23.2) | 212 (20.7) | 214 (19.9) | < 0.001 |
| Non-Hispanic white | 336 (32.8) | 472 (43.8) | 472 (48.1) | 520 (50.8) | 576 (53.4) | |
| Hispanic | 356 (34.8) | 286 (26.6) | 229 (23.3) | 247 (24.1) | 251 (23.3) | |
| Asian, other, unknown, or more than one race | 54 (5.3) | 68 (6.3) | 53 (5.4) | 45 (4.4) | 37 (3.4) | |
| BMI at labor and delivery admission - kg/m2 § | 31.6 (28–36.6) | 30.9 (27.5–35.1) | 30.7 (27.5–34.8) | 30 (27.3–34.6) | 30 (27–33.9) | < 0.001 |
| Married or living with partner | 550 (53.7) | 611 (56.7) | 612 (62.3) | 645 (63.0) | 685 (63.5) | < 0.001 |
| Employment status § | < 0.001 | |||||
| Full-time | 297 (29.1) | 412 (38.4) | 416 (42.4) | 446 (43.7) | 514 (47.9) | |
| Part-time | 114 (11.2) | 140 (13.1) | 122 (12.4) | 130 (12.7) | 125 (11.6) | |
| None | 610 (59.8) | 520 (48.5) | 444 (45.2) | 445 (43.6) | 435 (40.5) | |
| Private insurance § | 334 (32.6) | 479 (44.5) | 464 (47.3) | 523 (51.1) | 583 (54.1) | < 0.001 |
| Smoked cigarettes | 95 (9.3) | 90 (8.4) | 75 (7.6) | 66 (6.5) | 63 (5.8) | 0.02 |
| Drank alcohol § | 37 (3.6) | 51 (4.7) | 43 (4.4) | 37 (3.6) | 38 (3.5) | 0.51 |
| Method of conception was ovulation induction, artificial insemination or in vitro fertilization | 23 (2.3) | 28 (2.6) | 19 (1.9) | 25 (2.4) | 36 (3.3) | 0.32 |
| Study group assignment was induction of labor | 488 (47.7) | 517 (48.0) | 513 (52.2) | 539 (52.6) | 584 (54.2) | 0.006 |
| Labor & delivery variables | ||||||
| Type of labor | 0.08 | |||||
| Spontaneous | 132 (12.9) | 161 (15.0) | 149 (15.2) | 178 (17.4) | 150 (13.9) | |
| Spontaneous, augmented | 242 (23.6) | 266 (24.7) | 246 (25.1) | 270 (26.4) | 281 (26.1) | |
| Induced | 650 (63.5) | 650 (60.4) | 587 (59.8) | 576 (56.3) | 647 (60.0) | |
| Successful delivery type | < 0.001 | |||||
| Spontaneous vaginal | 586 (57.2) | 749 (69.6) | 714 (72.7) | 816 (79.7) | 890 (82.6) | |
| Forceps / vacuum vaginal | 84 (8.2) | 86 (8.0) | 93 (9.5) | 86 (8.4) | 75 (7.0) | |
| Cesarean | 354 (34.6) | 242 (22.5) | 175 (17.8) | 122 (11.9) | 113 (10.5) | |
| Overall pain § | 8.0 (6.0–9.5) | 7.0 (5.0–9.0) | 6.5 (5.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–7.5) | < 0.001 |
| Major maternal complications composite | 26 (2.5) | 28 (2.6) | 16 (1.6) | 9 (0.9) | 11 (1.0) | 0.003 |
| Neonatal variables | ||||||
| Birthweight - grams | 3343 (3050–3625) | 3350 (3080–3605) | 3345 (3080–3600) | 3355 (3073–3638) | 3319 (3060–3590) | 0.62 |
| Neonatal intermediate or intensive care unit admission | 155 (15.1) | 139 (12.9) | 113 (11.5) | 106 (10.4) | 109 (10.1) | 0.002 |
| Perinatal death or severe neonatal complications composite | 64 (6.3) | 57 (5.3) | 44 (4.5) | 37 (3.6) | 48 (4.5) | 0.06 |
Abbreviations: BMI, body mass index.
Data are n (%) or median (interquartile range) unless otherwise specified.
LAS score range in each quintile: 1st quintile 34–138, 2nd quintile 139–159, 3rd quintile 160–172, 4th quintile 173–184, 5th quintile 185–203.
Based on chi-square or Kruskal-Wallis tests.
Participant reported race or ethnicity group.
Number of missing values: BMI (28), employment status (15), private insurance (1) ,drank alcohol (1), and overall pain(60).
Table 3 shows the unadjusted results for the breastfeeding outcome. Being in the lowest two quintiles of the LAS score (ie, less perceived control during labor) was associated with a decreased likelihood of a woman exclusively breastfeeding as compared with exclusively bottle feeding (odds ratio [OR] 0.58, 95% confidence interval [CI] 0.47–0.71 for the first quintile, OR 0.80, 95% CI 0.65–0.97 for the second quintile). Similarly, when the LAS was modeled as a continuous variable, a higher LAS score was associated with a greater likelihood of a woman being in the exclusive breastfeeding group (OR = 1.007, 95% CI 1.005 – 1.01). The LAS score was not associated with a woman being in the breast and bottle group compared with the exclusive bottle feeding group.
Table 3.
Unadjusted Model Results for Infant Feeding Method*
| Breastfeeding† | Breastfeeding & Formula† | |
|---|---|---|
| Odds Ratio (95%CI) | Odds Ratio (95%CI) | |
| LAS score 6–96 hours after delivery | ||
| 1st quintile | 0.58 (0.47–0.71) | 0.97 (0.79–1.19) |
| 2nd quintile | 0.80 (0.65–0.97) | 0.88 (0.72–1.09) |
| 3rd quintile | 0.85 (0.69–1.04) | 0.97 (0.78–1.21) |
| 4th quintile | 0.96 (0.78–1.18) | 0.97 (0.78–1.20) |
| 5th quintile | (ref) | (ref) |
Abbreviations: LAS, labor agentry scale.
Multinomial logistic model.
Compared with exclusive formula feeding.
In the multivariable adjusted model, perceived control during labor was no longer associated with exclusive breastfeeding (Table 4). The association between the bottom two quintiles of the LAS score and infant feeding method became non-significant when adjusting for either race and ethnicity or private insurance. Rather, maternal demographic characteristics and behaviors, such as maternal race, ethnicity, BMI, smoking, age, marital status, insurance type were associated with infant feeding method. Additional significant factors included type of delivery and neonatal intermediate or intensive care unit admission.
Table 4.
Multivariable Model Results for Infant Feeding Method*
| Breastfeeding† | Breastfeeding & Formula† | |
|---|---|---|
| Odds Ratio (95%CI) | Odds Ratio (95%CI) | |
| LAS score 6–96 hours after delivery | ||
| 1st quintile | 0.95 (0.74–1.21) | 1.07 (0.86–1.34) |
| 2nd quintile | 1.02 (0.81–1.28) | 0.96 (0.77–1.20) |
| 3rd quintile | 0.95 (0.75–1.20) | 1.03 (0.82–1.29) |
| 4th quintile | 1.01 (0.81–1.27) | 1.00 (0.80–1.25) |
| 5th quintile | (ref) | (ref) |
| Maternal age, per unit increase in years | 1.06 (1.04–1.08) | 1.06 (1.04–1.07) |
| Race or ethnic group | ||
| Black Non-Hispanic | 0.53 (0.42–0.66) | 0.84 (0.68–1.03) |
| Hispanic | 0.79 (0.63–0.98) | 2.00 (1.64–2.44) |
| Other / unknown / Asian | 1.07 (0.74–1.54) | 1.65 (1.15–2.36) |
| White Non-Hispanic | (ref) | (ref) |
| Maternal BMI at admission, per unit increase in kg/m2 | 0.95 (0.94–0.96) | 0.98 (0.97–0.99) |
| Married or living with partner | 2.35 (1.96–2.82) | 1.48 (1.26–1.75) |
| Private insurance | 2.38 (1.97–2.88) | 1.47 (1.22–1.77) |
| Smoke cigarettes | 0.38 (0.28–0.53) | 0.48 (0.36–0.63) |
| Successful delivery type | ||
| Forceps / vacuum vaginal | 1.42 (1.04–1.93) | 0.79 (0.58–1.07) |
| Spontaneous vaginal | 1.34 (1.09–1.65) | 0.93 (0.77–1.11) |
| Cesarean | (ref) | (ref) |
| Neonatal intermediate or intensive care unit admission | 0.64 (0.51–0.81) | 0.82 (0.66–1.01) |
Abbreviations: LAS, labor agentry scale; BMI, body mass index.
Multinomial logistic model includes all variables in the table.
Compared with exclusive formula feeding.
We conducted two sensitivity analyses assessing infant feeding method as a dichotomous variable. In the first analysis, we characterized infant feeding as exclusive breastfeeding versus all other methods. Once again, in unadjusted analyses, being in the lowest quintile of the LAS scale, as compared with the highest quintile, was associated with decreased likelihood of exclusive breastfeeding (unadjusted OR = 0.58, 95% CI = 0.48 – 0.70). Once confounding variables were included, LAS quintile was no longer significant. In the second sensitivity analysis, infant feeding was dichotomized as any breastfeeding versus exclusive bottle feeding. While being in the lowest two quintiles of the LAS score was associated with decreased likelihood of any breastfeeding in unadjusted models (unadjusted OR = 0.75, 95% CI = 0.63 – 0.90 for the lowest quintile, unadjusted OR = 0.84, 95% CI = 0.70 – 0.996 for the second lowest quintile), these results were no longer significant in adjusted models.
Discussion:
Among this group of nulliparous women, perceived control during labor as measured by the LAS was not associated with infant feeding method (exclusive breastfeeding, breast and bottle feeding, or exclusive bottle feeding) at the postpartum visit. Rather, whether a woman was breastfeeding was associated with demographic characteristics such as race, age, ethnicity, insurance status, and marital status, as well as variables pertaining to the pregnancy including mode of delivery and whether the neonate went to the intermediate or intensive care unit. These findings are consistent with those from other studies on breastfeeding.21–23
While there are scant data that assessed labor agentry per se as an independent predictor of breastfeeding, other work evaluated the influence of intrinsic personality characteristics and internal motivation on breastfeeding rates. Henshaw et. al.24, for instance, found that breastfeeding self-efficacy (confidence in one’s ability to successfully breastfeed) measured during the postpartum period of the delivery hospitalization was positively associated with exclusive breastfeeding at six months’ postpartum. This finding persisted even when accounting for extrinsic factors such as maternal employment arrangements. Other work suggests that seemingly ‘intrinsic’ factors, such as breastfeeding efficacy, are themselves influenced by factors such as health care worker support for breastfeeding and delays in initiating breastfeeding after delivery.25–26
Our study did not show support for an association between increased feelings of perceived control during childbirth as measured by the Labor Agentry Scale and breastfeeding, but this may be either because there is no association, or because the LAS score itself is influenced by factors also associated with breastfeeding, such as mode of delivery. In terms of clinical significance, while increasing a woman’s feelings of control during labor may be important for other reasons, increasing self-efficacy in labor may not necessarily increase breastfeeding rates.
Many recent policy recommendations from medical societies and other researchers have focused on removing external barriers to breastfeeding, such as increasing paid maternal leave from work, work site support for pumping, and baby-friendly hospital initiatives that decrease logistical barriers to breastfeeding.27 In addition to addressing systematic barriers, whether measures such as increased medical staff-driven education and support can lead to increased feelings of control over breastfeeding and, ultimately, higher rates of breastfeeding, remains an area for future research.
Strengths of this study include a large, racially diverse, sample of nulliparous pregnant women drawn from multiple institutions, increasing the external validity of the study. Other strengths include the use of a validated scale to measure agentry during labor, as well as close follow up using standardized questionnaires regarding breastfeeding behavior. This study also has several limitations. Infant feeding behavior is self-reported, and may be subject to bias due to women over-reporting the socially desirable behavior of breastfeeding.28 There may be selection bias in which women chose to participate in the ARRIVE trial and fill out the LAS survey. As noted in Table 4, there were some significant differences between women who had complete data on the LAS survey and infant feeding method and those women who did not in terms of race, ethnicity, marital status, employment status, insurance, treatment group, and pain score, which may have biased the results of the study through sample selection bias. We were unable to control for other factors that may be associated with both the LAS and infant feeding, such as household income, maternal employment in the immediate postpartum period, and other aspects of the delivery hospitalization, such as medical staff support for breastfeeding. All women in this study were nulliparous; labor agentry may have a different effect in multiparous women, particularly once previous experience with breastfeeding is taken into account. Data on infant feeding method were collected 4–8 weeks after delivery; had data been collected over a longer time period, responses may have differed. Finally, all associations presented are correlational only; causality cannot be implied.
In conclusion, in this sample of nulliparous women, maternal sense of control in labor, as measured by the Labor Agentry Scale, was not associated with infant feeding method at 4–8 weeks postpartum.
Supplementary Material
Authors’ Data Sharing Statement.
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? Not available.
What other documents will be available? Not available.
When will data be available (start and end dates)? Not applicable.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
Acknowledgements:
The authors thank Gail Mallett, R.N., M.S., C.C.R.C. and Kim Hill, R.N., B.S.N. for protocol development and coordination between clinical research centers; Lindsay Doherty, M.S. for protocol and data management; and William A. Grobman, M.D., M.B.A., Elizabeth A. Thom, Ph.D., and Madeline M. Rice, Ph.D. for protocol development and oversight.
Funding: This work was supported by grants (HD40512, HD36801, HD27869, HD34208, HD68268, HD40485, HD40500, HD53097, HD40560, HD40545, HD27915, HD40544, HD34116, HD68282, HD87192, HD68258, HD87230) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Center for Advancing Translational Sciences (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure
Sindhu K. Srinivas disclosed receiving funding from law firms for expert witness work. The other authors did not report any potential conflicts of interest.
Presented at the 2019 Annual Scientific Meeting of the Society for Maternal-Fetal Medicine, February 11–16, 2019, Las Vegas, NV.
References:
- 1.Jarlenski MP, Bennett WL, Bleich SN, Barry CL, Stuart EA. Effects of breastfeeding on postpartum weight loss among U.S. women. Prev Med. 2014;69:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and Endometrial Cancer Risk: An Analysis From the Epidemiology of Endometrial Cancer Consortium. Obstet Gynecol. 2017;129(6):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansstein FV. The Impact of Breastfeeding on Early Childhood Obesity: Evidence From the National Survey of Children’s Health. Am J Health Promot. 2016;30(4):250–258. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JMD, Tanabe K, Moon RY, et al. Duration of Breastfeeding and Risk of SIDS: An Individual Participant Data Meta-analysis. Pediatrics. 2017;140(5). [DOI] [PubMed] [Google Scholar]
- 5.Bartick MC, Jegier BJ, Green BD, Schwarz EB, Reinhold AG, Stuebe AM. Disparities in Breastfeeding: Impact on Maternal and Child Health Outcomes and Costs. J Pediatr. 2017;181:49–55 e46. [DOI] [PubMed] [Google Scholar]
- 6.Louis-Jacques A, Deubel TF, Taylor M, Stuebe AM. Racial and ethnic disparities in U.S. breastfeeding and implications for maternal and child health outcomes. Semin Perinatol. 2017;41(5):299–307. [DOI] [PubMed] [Google Scholar]
- 7.Ogbuanu C, Glover S, Probst J, Liu J, Hussey J. The effect of maternity leave length and time of return to work on breastfeeding. Pediatrics. 2011;127(6):e1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binour LM, Szaro JM. Employer-based programs to support breastfeeding among working mothers: a systemic review. Breastfeed Med. 2017. April;12:131–141. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs AJ, Mannion CA, McDonald SW, Brockway M, Tough SC. The impact of cesarean section on breastfeeding initiation, duration, and difficulties in the first four months postpartum. BMC Pregnancy Childbirth. 2016. April 26;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes M, Trocado V, Carlos-Alves M, Arteiro D, Pinheiro P. Intrapartum synthetic oxytocin and breastfeeding: a retrospective cohort study. J Obstet Gynaecol. 2018. August; 38(6):745–749. [DOI] [PubMed] [Google Scholar]
- 11.Hinic K Predictors of breastfeeding confidence in the early postpartum period. J Obstet Gynecol Neonatal Nurs. 2016. Sep-Oct; 45(5)649–660. [DOI] [PubMed] [Google Scholar]
- 12.Hodnett ED, Simmons-Tropea DA. The labor agentry scale: psychometric properties of an instrument measureing control during childbirth. Res Nurs Health. 1987. October;10(5):301–310. [DOI] [PubMed] [Google Scholar]
- 13.Adewuya AO, Ologun YA, Ibigbami OS. Post-traumatic stress disorder after childbirth in Nigerian women: prevalence and risk factors. BJOG. 2006. March;113(3):284–288. [DOI] [PubMed] [Google Scholar]
- 14.Grajeda R, Perez-Escamilla R. Stress during labor and delivery is associated with delayed onset of lactation among urban Guatemalan women. J Nutr. 2002. October;132(10):3055–3060. [DOI] [PubMed] [Google Scholar]
- 15.Ford E, Ayers S, Wright DB. Measurement of maternal perceptions of support and control in birth (SCIB). J Womens Health (Larchmt). 2009. February;18(2):245–252. [DOI] [PubMed] [Google Scholar]
- 16.Garthus-Niegel S, Horsch A, Ayers S, Junge-Hoffmeister J, Weidner K, Eberhard-Gran M. The influence of postpartum PTSD on breastfeeding: a longitudinal population-bsed study. Birth. 2018. June;45(2):193–201. [DOI] [PubMed] [Google Scholar]
- 17.Brownell E, Howard CR, Lawrence RA, Dozier AM. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012. October;161(4):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinic K Predictors of breastfeeding confidence in the early postpartum period. J Obstet Gynecol Neonatal Nurs. 2016. Sep-Oct;45(5):649–660. [DOI] [PubMed] [Google Scholar]
- 19.Grobman WA, Rice MM, Reddy UM, et al. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N Engl J Med. 2018;379(6):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludington E, Dexter F. Statistical analysis of total labor pain using the visual analog scale and application to studies of analgesic effectiveness during child-birth. Anesth Analg. 1998;87: 723–7. [DOI] [PubMed] [Google Scholar]
- 21.Singh GK, Kogan MD, Dee DL. Nativity/immigrant status, race/ethnicity, and socioeconomic determinants of breastfeeding initiation and duration in the United States, 2003. Pediatrics. 2007. February;119 Sppl 1:S38–46. [DOI] [PubMed] [Google Scholar]
- 22.Dagher RK, McGovern PM, Schold JD, Randall XJ. Determinants of breastfeeding initiation and cessation among employed mothers: a prospective cohort study. BMC Pregnancy Childbirth. 2016. July 29;16(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regan J, Thompson A, DeFranco E. The influence of mode of delivery on breastfeeding initiation in women with a prior cesarean delivery: a population-based study. Breastfeed Med. 2013. April; 8:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henshaw EJ, Fried R, Siskind E, Newhouse L, Cooper M. Breastfeeding self-efficacy, mood, and breastfeeding outcomes among primiparous women. J Hum Lact. 2015. August; 31(3):511–8. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Gao LL, Ip WY, Sally Chan WC. Predictors of breastfeeding self-efficacy in the immediate postpartum preiod: a cross-sectional study. Midwifery. 2016. October; 41:1–8. [DOI] [PubMed] [Google Scholar]
- 26.Dennis CL. Identifying predictors of breastfeeding self-efficacy in the immediate postpartum period. Res Nurs Health. 2006. August; 29(4):256–68. [DOI] [PubMed] [Google Scholar]
- 27.Patnode CD, Henninger ML, Senger CA, Perdue LA, Whitlock EP. Primary care interventions to support breastfeeding: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016. October 25; 316(16):1694–1705. [DOI] [PubMed] [Google Scholar]
- 28.Greiner T Exclusive breastfeeding: measurement and indicators. Int Breastfeed J. 2014. October 20; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


