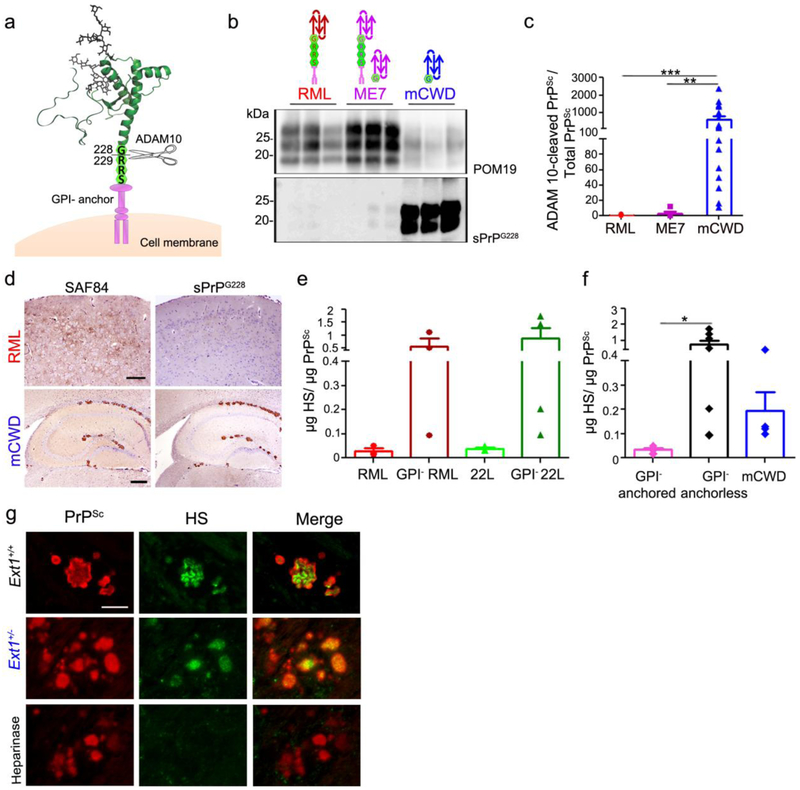
Fig. 4.
ADAM10-cleaved and full length GPI-anchorless prions bind HS. a Schematic representation of ADAM10 cleavage at mouse PrP residue 228 shows the release of shed PrP lacking the GPI-anchor and three C-terminal amino acid residues (RRS). b Immunoblots of brain homogenate from prion-infected Ext1+/+ mice using POM19 antibody (PrP) and sPrPG228 antibody (ADAM10-cleaved PrP). c Ratios of ADAM10-cleaved PrPSc relative to total PrPSc reveal significantly higher levels of ADAM10-cleaved PrP in mCWD as compared to the RML and ME7 strains. d Brain immunolabelled for PrP with SAF84 (amino acids 163–169 of mouse PrP) and sPrPG228 antibodies reveals all mCWD plaques, but few diffuse RML aggregates, are labelled by sPrPG228. e Quantification of HS bound to GPI-anchored and –anchorless RML and 22L prions by LC-MS, and f a grouped comparison of HS bound to GPI-anchored prions (RML and 22L) versus GPI-anchorless prions (GPI− RML, GPI− 22L) and ADAM10-cleaved mCWD shows that full length, GPI-anchorless prions and ADAM10-cleaved prions (mCWD) bind more HS than their GPI-anchored counterparts. g Dual immunostaining of mCWD-infected brain sections for PrP and HS shows parenchymal prion plaques in the corpus callosum label strongly for HS. Pre-treating brain sections with heparinases abolished HS labelling of plaques. Scale bars = 200 μm and 500 μm for upper and lower panel (panel d) and 25 μm (panel g). *P< 0.05, **P< 0.01 and ***P< 0.001, Wilcoxon rank sum test (panel c) and one-way ANOVA with Tukey’s post test (panel f).