Abstract
Background:
Radiosensitization using nanoparticles is proposed as a novel strategy for treatment of different cancers. Superparamagnetic iron oxide nanoparticles (SPIONs) have been reported to enhance effects of radiotherapy in several researches.
Objective:
The objective of this research is to investigate the radiosensitization properties of polyglycerol coated SPIONs (PG-SPIONs) on U87-MG cancer cells.
Material and Methods:
In this experimental study, polyglycerol coated SPIONs were synthesized by thermal decomposition method and characterized by FTIR, TEM and VSM analysis. Cellular uptake of nanoparticles by cells was examined via AAS. Cytotoxicity and radiosensitization of nanoparticles in combination with radiation were evaluated by MTT and colony assay, respectively.
Results:
Mean size of nanoparticles was 17.9±2.85 nm. FTIR verified SPIONs coating by Polyglycerol and VSM showed that they have superparamagnetic behaviour. Viability significantly (P < 0.001) decreased at concentrations above 100µg/ml for SPIONs but not for PG-SPIONs (P > 0.05). Dose verification results by TLD for doses of 2 and 4 Gy were 2±0.19 and 4±0.12 Gy respectively. The combination index for all situations was less than 1 and the effect is antagonism.
Conclusion:
However, PG-SPIONs combination with 6 MV X-ray reduced survival of U87-MG cells compared to radiation alone but the effect is antagonism.
Keywords: Polyglycerol, X-Rays, Magnetite Nanoparticles
Introduction
Combination of radiotherapy with nanoparticles as radiosensitizer agents is proposed for treatment of cancers in different studies [ 1 - 6 ]. Radiotherapy plays an important role in treatment of cancers. About fifty percent of cancers are currently treated by this modality [ 7 ]. Radiotherapy goal is to kill all cancer cells with no damage to soft tissues [ 8 ]. Many efforts have been made in radiation dose delivery techniques to achieve this goal, but there are still inefficiencies which prevent some cancers such as Glioblastoma Multiforme (GBM) being treated to the full [ 9 , 10 ]. GBM is the most malignant and frequent primary malignancy of the brain which has remained as a clinical unsolved problem [ 11 - 14 ]. The prognosis for GBM is really poor [ 15 , 16 ].
A standard method for treatment of GBM is currently surgery followed by radiotherapy and concurrent chemotherapy. However, there are some limitations such as remaining of residual malignant cancer cells in the tumor site after surgery, insufficient dose of radiation to the cancer cells and resistance of tumor cells to radiotherapy [ 17 ]. Radiation dose for treatment of GBM is usually 60 Gy delivered in 30 fractions [ 18 ]. This dose is not sufficient to destroy all cancer cells and increasing dose is generally associated with a rise in soft tissues injures [ 19 , 20 ].
Among different ways which are suggested to overcome these limitations, nanoparticles have attracted a lot of attention to themselves [ 21 ]. Superparamagnetic iron oxide nanoparticles (SPIONs) seem to be a good option for treatment of GBM due to their capability for drug delivery, diagnosis, radiosensitization, biocompatibility and selective targeting under the direction of an external magnetic field [ 22 - 25 ]. SPIONs were reported to enhance the effect of radiation on cancer cells in several studies [ 3 , 26 , 27 ]. Klein et al. studied the effect of combining SPIONs with X-ray on MCF-7 cells and expressed that citrate coated SPIONs are excellent radiosensitizers which enhance the generation of reactive oxygen spices (ROS) about 240% [ 27 ]. Iron atomic number is relatively high compared to soft tissue which causes increasing X-ray absorption and radiosensitization of cancer cells [ 28 ]. Some damage to cancer cells has been reported by iron oxide nanoparticles combination with ionizing radiation [ 26 ].
Coating SPIONs by a biocompatible material has several advantages such as prevention of agglomeration with itself [ 29 - 31 ]. Hyperbranched Polyglycerol (HPG) is proposed as a suitable coating agent for SPIONs [ 32 , 33 ]. It exhibits the great promise in the area of nanoparticles coating material even superior to PEG as reported by Deng et al [ 34 ]. In this study, we aimed to synthesize Polyglycerol coated SPIONs (PG-SPIONs) and examine their cytotoxicity and synergistic effects between megavoltage X-ray combination and these nanoparticles in order of cancer treatment.
Material and Methods
Nanoparticles synthesis and characterization
In this experimental study, nanoparticles were synthesized based on polyol process [ 32 ]. 0.53 gr of Fe (acac)3 (Merk) was mixed with 30 ml TREG(PVT.LTD). After stirring under gentle pressure of nitrogen the mixture gradually heated until reflux temperature [ 35 ]. When reflux is completed, solvent color goes to dark black. Then sediment is freeze-dried (VaCo5 ZIRBUS) [ 36 ]. To coat, 0.5 ml Glycidol monomer was mixed with 15 mg dried SPIONs while stirring at constant temperature 284ºF for 1200 minutes [ 32 ]. PG-SPIONs sediment then dialyzed using a dialyze bag with a cut-off of 12 kDa.
Transmission microscope (TEM) (Leo 912 AB) images were taken from PG-SPIONs to determine their size and morphology. A program was written on MATLAB(R 2009a) software in order of getting size distribution and mean size of nanoparticles using TEM images. Fourier transform infrared (FT-IR) (JACSO 6300 FT-IR spectrometer) with a resolution of 4 cm-1 was taken from PG coated SPIONs to show what groups are included in them. A little amount of SPIONs and PG coated SPIONs were mixed with potassium bromide (KBr) to form a tablet for analysis. Magnetic property of SPIONs is important for biological applications. SPIONs treat such as they become magnetic in the presence of a magnetic field and loss their magnetic properties in the absent of it immediately [ 37 , 38 ]. To define magnetic properties of nanoparticles, vibrating sample magnetometer (VSM) (Meghnatis daghigh kavir kashan) was taken. About 100 mg NPs was used for this analysis.
Biological tests
U87-MG cells line was obtained from Pasteur institute of Iran. The cells were grown in T-25 flasks containing culture medium with high glucose DMEM, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Flasks were maintained in an incubator (BINDER) under 5% CO2 pressure at 37ºC temperature. When cells well grew and reached to a suitable confluence, they were separated from the flask floor by 0.25 % trypsin/EDTA solution and were counted for biological tests.
MTT Assay
MTT test was used to examine the cytotoxicity of SPIONs and PG-SPIONs on U87-MG cells. First, the cells were cultured in 12-well plates at the concentrations of 20000 cells in each well. Five groups were chosen to examine cytotoxicity of nanoparticles at different concentrations including 5, 25, 50, 100 and 200µg/ml and one group was left as a control group without any nanoparticles. For each concentration, three wells were cultured. Nanoparticles contained medium were filtered through 0.2 µl filter before addition to wells. After removing nanoparticle-free medium, U87-MG cells were washed twice with PBS and incubated for 24, 48 and 72 hours after adding SPIONs or PG-SPIONs containing medium. After removing a consumed medium, 200 µl MTT solution (nutrient medium containing 5mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added to each well and remained in an incubator for 4 hours at 37ºC. Next, the supernatant was carefully taken and 440 µl DMSO was used to dissolve the formazan salt in each well. Cells viability was examined by measuring light absorbance of the solution using 96-well plates. Reading was performed by a microplate reader (BIO-RAD Model 680) at the wavelength 570nm. The percentage of optical density reading for treated cells with nanoparticles divided by the same for untreated cells was considered to be the cells viability.
For cytotoxicity evaluation of 6 MV X-ray on U87-MG cancer cells, they were irradiated using Anchor accelerators located at Milad Hospital of Isfahan. A number of 20000 cells per each well (24-well plate) were exposed to doses of 1, 2, 4 and 6 Gy in a 20×20 cm2 field at the percentage depth dose of 97.8. Dose calculation was performed using PDD table of the accelerator and 5 cm thickness backscatter. Plexiglas acrylic sheets were placed under plates to ensure that the dose is uniform through the irradiated field. MTT assay was performed such as that done for SPIONs and PG-SPIONs. In order to ensure correct radiation dose delivering to cells, dose verification was performed for doses of 2 and 4 Gy using thermoluminescent dosimeter (TLD-100). Two TLDs were embedded in water resistance bags and placed in water filled well shown in (Figure 1) under the field of radiation with the same conditions for cell containing plates. Kruskal-Wallis test was used for statistical analysis of the MTT test results.
Figure 1.

Dose verification using TLD-100 dosimeters.
Colony assay
To prevent cancer cells from recurring, killing of those cells which can form a colony is very important [ 39 ]. Colony assay as a gold standard method is usually performed to investigate survival of irradiated or treated cancer cells [ 40 - 42 ]. A number of 50000 cells per each well (12-well plate) were treated by PG-SPIONs at the concentration of 100µg/ml after removing the cultured medium. Cells were then exposed to doses of 1, 2, 4 and 6 Gy at room temperature. X-ray irradiation was performed by 6 MV X-ray with output of 1cGy/MU. Irradiated cells then were carefully counted and numbers of 1000 or 2000 cells were cultured in 60 mm petri dishes in triplicate. After irradiation, cells were left in an incubator for 14 days. After removing consumed medium, petri dishes were washed twice by PBS. Colonies were fixed by formaldehyde and stained with 5% crystal violet in PBS. Colonies which had more than 50 cells were counted and a survival fraction was determined for each group by the equation 1 [ 41 ].
(1)
Which PE (plating efficiency) is the ratio of the number of colonies to the number of cells seeded (equation 2):
(2)
To determine combination effects between radiation and nanoparticles, Chou analysis was performed using compusyn software [ 43 ].The combination index (CI) was used to determine if the effect is synergistic or antagonistic. CI less than 1 and greater than 1 indicates synergistic and antagonistic, respectively.
Cellular uptake of nanoparticles determined using atomic absorption spectroscopy (AAS). Cells were first cultured and washed twice with PBS. A numbers of 2×106 cells were counted and transferred to 60 mm petri dishes and placed in an incubator for 24 hours. PG-SPIONs at concentrations 1, 2 and 3 mg/ml were added to the cells and placed in an incubator for 2 days, then washed with PBS three times and trypsinized.
After centrifuging, the remaining cells in bottom of falcons were used for AAS. 1.5 ml Aqua regia were added to the cells in each falcon and the final volume of deionized water increased to 5 ml. The falcons were placed in a water bath under 45ºC temperature for 4 h and the digested homogenate was centrifuged at 19400g. The supernatant was used for AAS. The measurements were performed using PERKIN-ELMER AAS following calibration process.
Results
Nanoparticles characterization
PG-SPIONs shape and size distributions are shown in Figure 2a and b, respectively. Mean diameter of PG coated SPIONs was 17.9 ± 2.85 nm. They have a spherical morphology.
Figure2.
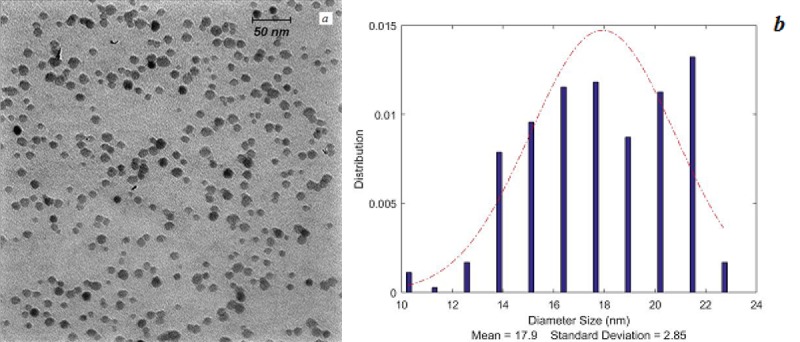
(a) Transmission electron microscopy of PG-SPIONs and (b) their size distributions.
Figure 3 shows FTIR spectrum of undialyzed PG-SPIONs. The presence of the peaks related to C-O-C and C-H bonds is clearly seen on the spectrum. In this case, SPIONs have been coated with polyglycerol successfully. Peak related to glycidol has been removed from FTIR spectrum by the dialyze bag.
Figure3.
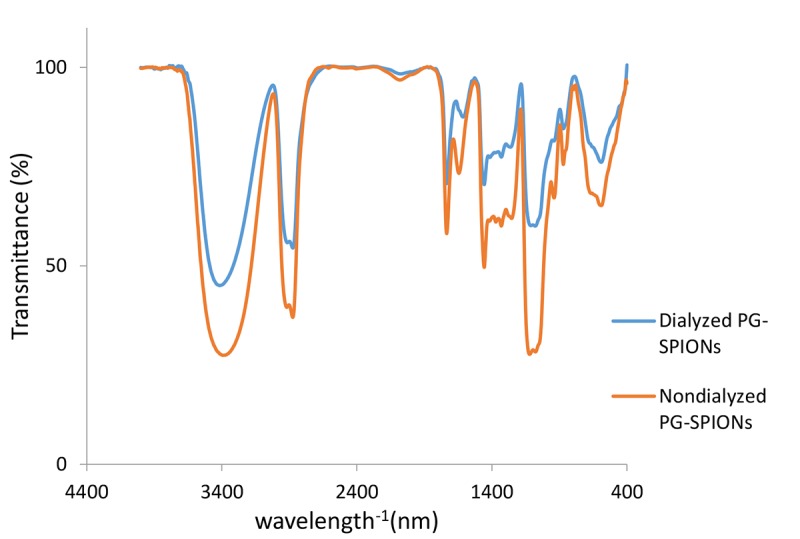
Dialyzed and undialyzed PG-SPIONs FTIR.
Figure 4 shows magnetic properties of nanoparticles. Saturation magnetization for SPIONs is greater than PG-SPIONs [ 20 ].
Figure4.
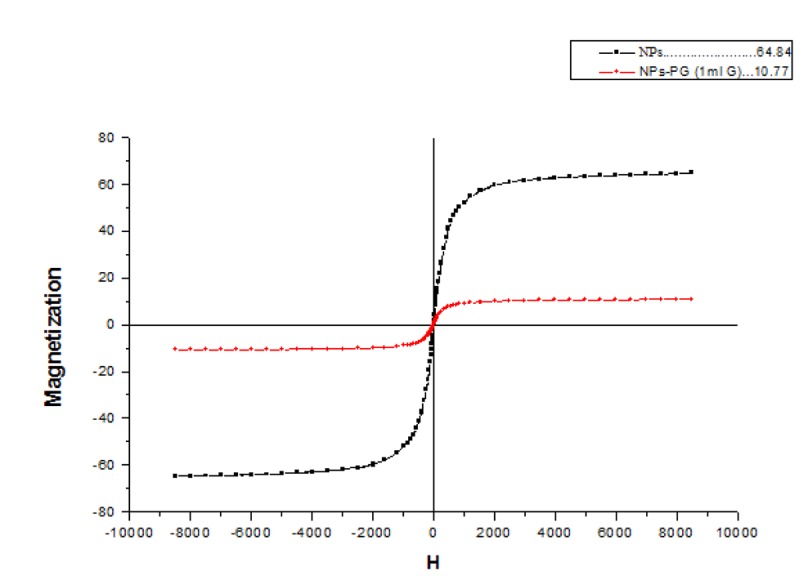
Magnetic properties of nanoparticles.
Viability tests
U87-MG cells pictures which have taken by an inverted microscope (Nikon Eclipse - TS100) with different magnifications are shown in Figure 5 from part (a) to (f). Cells degradation is seen in this figure part c and d. Cells colony is obviously shown in part e and f.
Figure5.
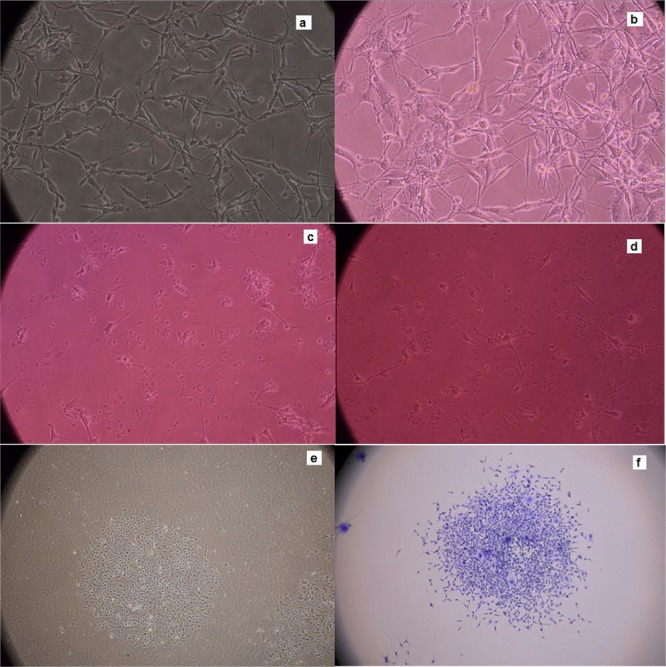
U87-MG cells pictures (a) 48 h post culturing (b) after treating with PG-SPIONs (c) exposed to 6 Gy radiation dose (d) treated with PG-SPIONs and exposed to dose 6 Gy radiation dose (e) colony formed and (f) stained colonies for counting.
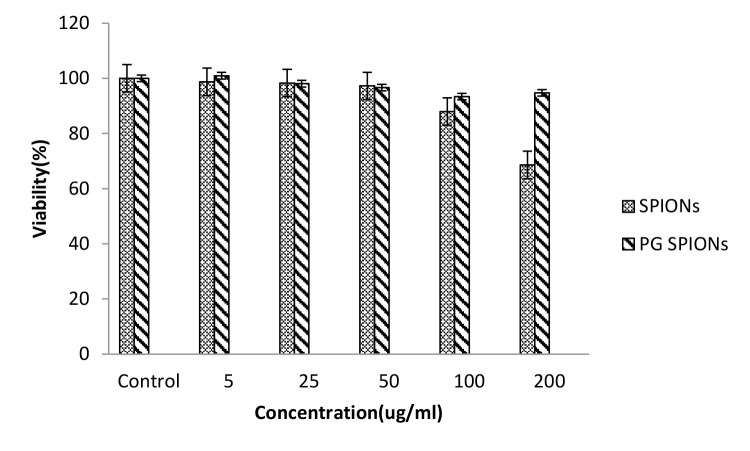
Cytotoxicity of treated cells with SPIONs, PG-SPIONS, 6 MV X-ray and their combination were evaluated by MTT assay. Figures 6-8 show viability percent of U87-MG cells treated with SPIONs and PG-SPIONS incubated for 24, 48 and 72 hours, respectively. Viability significantly (P < 0.001) decreases at concentrations above 100µg/ml for SPIONs in all times but it does not differ for PG-SPIONs. (P > 0.05).
Figure6.
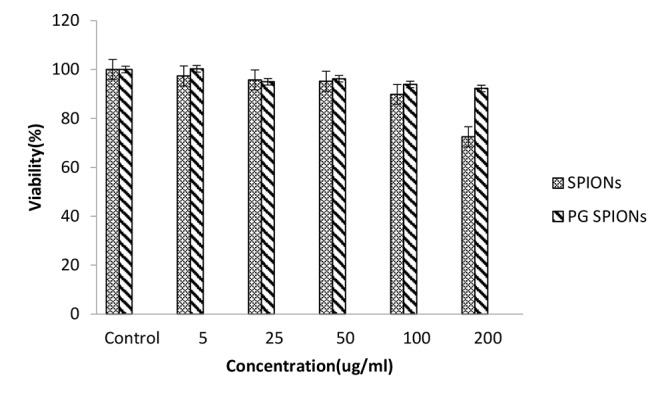
Measured viability for U87-MG cancer cells treated with SPIONs and PG-SPIONs for incubation time of 24h.
Figure7.
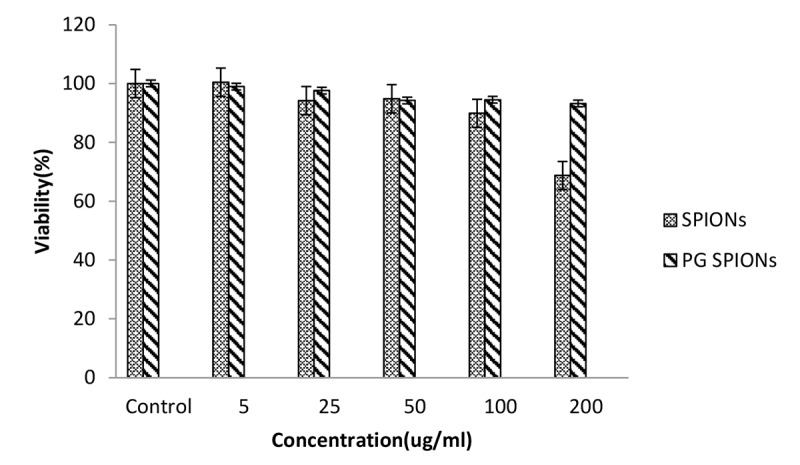
Measured viability for U87-MG cancer cells treated with SPIONs and PG-SPIONs for incubation time of 48h.
Figure8.
Measured viability for U87-MG cancer cells treated with SPIONs and PG-SPIONs for incubation time of 72h.
Figure 9 shows viability percent of U87-MG cells irradiated with doses of 1, 2, 4 and 6 Gy from 6 MV X-ray. Viability decreases significantly (P < 0.001) with increasing radiation dose. Dose verification results by TLD for irradiation of 2 and 4 Gy to cancer cells were 2±0.19 and 4±0.12 Gy respectively.
Figure9.
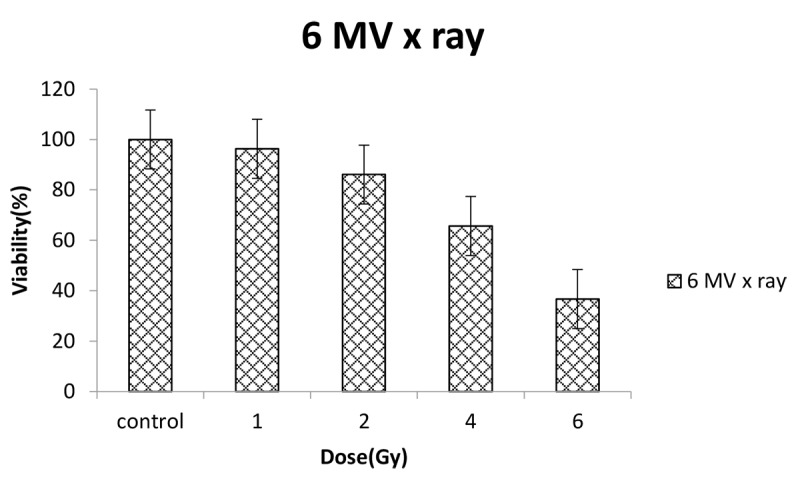
Measured viability for U87-MG cancer cells exposed to doses of 1, 2, 4 and 6 Gy from 6 MV X rays.
Colony assay results are shown by dose response curves which consist of a vertical axis with a logarithmic scale to determine a survival fraction and a horizontal axis defining radiation doses. The survival curve for combination of 6 MV X-ray and PG-SPIONs at the concentration 100 ug/ml is shown in Figure 10. Reduction in the survival fraction by dose increasing for combination of nanoparticles with radiation is greater than radiation alone but it is not significant (P>0.05).
Figure10.
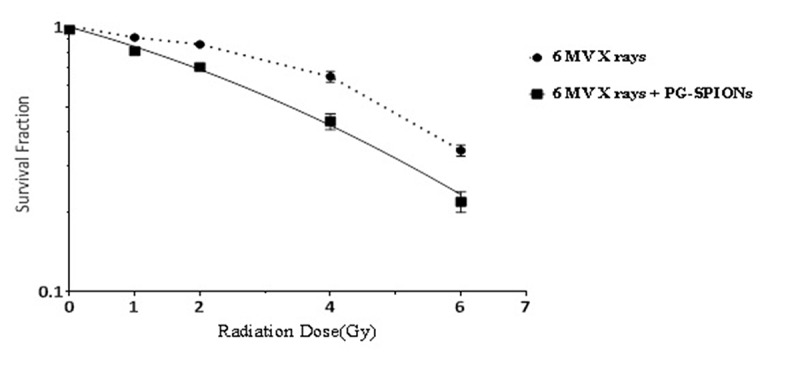
Survival curve for U87-MG cancer cells treated with combination of 6 MV X rays and PG-SPIONs at concentration of 100ug/ml.
Results of combination index calculation of PG-SPIONs combination with 6 MV X-rayshowed that for all situations CI was less than 1 and the effect is antagonistic.
AAS results are shown in Figure 11. Cellular uptake of nanoparticles increased with higher doses of nanoparticles (P < 0.05) but for all concentrations the uptake is relatively low.
Figure11.
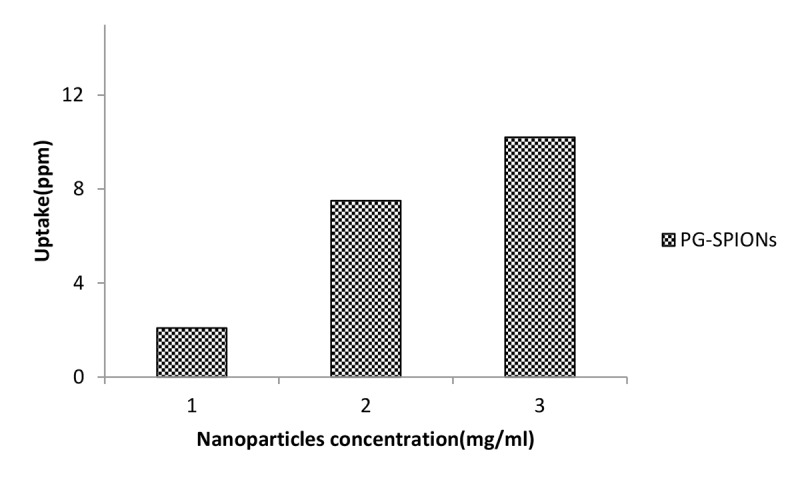
PG-SPIONs uptake by U87-MG cancer cells treated with doses of 1, 2 and 3 mg/ml nanoparticles.
Discussion
In this study we were going to synthesize PG coated SPIONs in order to increase radiosensitivity of U87-MG cancer cells. Results showed that size and size distribution of our nanoparticles are suitable for biomedical applications. As expected, magnetization of PG-SPIONs decreased compared to SPIONs. This situation is seen at other researches [ 26 , 44 , 45 ]. This can be due to coating of SPIONs with Polyglycerol which reduces SPIONs concentration for analysis and magnetization consequently [ 46 ]. There is a good agreement between our results with Martin et al [ 45 ]. Strong peaks on FTIR spectrum in our study indicated successful coating of SPIONs by polyglycerol which already has been reported by Wang et al [ 47 ].
For uncoated SPIONs, cells viability significantly (P < 0.001) decreases at concentrations above 100µg/ml. This result is also reported in other similar studies [ 48 - 50 ]. Ankamwar et al, have reported that SPIONs at low concentrations have no cytotoxicity effects on U87-MG cancer cells but obvious cytotoxicity is seen at concentration of 100 ug/ml [ 48 ]. Entry of SPIONs into cancer cells leads to formation of reactive oxygen spices(ROS) such as H2O2, OH0 and anion superoxides (O2-) which in turn causes oxidative stress and toxicity in cells [ 51 ].Viability for U87-MG cells treated with PG-SPIONs is higher compared with SPIONs. Despite of SPIONs, viability did not decrease by increasing concentrations of PG-SPIONs (P > 0.001). In other studies cytotoxicity is not reported even for higher concentrations of PG-SPIONs [ 52 ]. Saatchi et al, examined cytotoxicity of PG coated gallium on HUVEC cancer cells and the results showed no significant toxicity effects at the concentration of 10 mg/ml [ 53 ]. Low uptake of PG-SPIONs by U87-MG cells can be attributed to the surface hydrophilicity of nanoparticles [ 47 ].
U87-MG cancer cells viability significantly (P < 0.001) decreased by increasing radiation doses. Radiation interaction with cancer cells can damage DNA directly or produce free radicals by radiolysis of water molecules that in turn is destructive and kills the cells [ 54 , 55 ]. Reactive oxygen spices (ROS) are generated as a result of reactions free radicals with biological molecules which cause oxidation of lipids, protein and DNA that induce apoptotic and necrotic cell death due to mitochondrial dysfunction [ 56 , 57 ].
SPIONs aggregations on cell membrane cause a membrane lipid peroxidation and improve radiosensitization [ 58 ]. Klein et al, expressed that production of ROS increased in SPIONs loaded with MCF-7 cells exposed to X-ray increased compared with cells only exposed to X-ray. The ROS production was explained to be related with release of iron ions into the cytosol where immediate chelation by citrate or adenosine phosphate will take place and active surface of nanoparticles as catalyst for the Haber-Weiss cycle [ 27 , 59 , 60 ]. ROS production increases with a rise in iron ions in cancer cells which in turn leads to more cellular cytotoxicity [ 1 ]. Synergy is that the effect of two agents given together is more effective than would be predicted based on their individual activity; If agent 1 alone reduces the surviving fraction to 0.5 and agent 2 used alone also reduces survival to 0.5, the combination effect will be synergistic if it is less than 0.25 [ 61 ]. Combination of PG-SPIONs with 6 MV X-ray reduced survival fractions of U87-MG cells compared to radiation alone but difference was not significant (P>0.05). Although radiosensitization of SPIONs alone and with different coating has been reported in different studies, but in our study the radiation effect enhancement property of these nanoparticles was not considerable. It may be related to the coating material of HPG. Results of MTT assay for PG-SPIONs confirm this because viability did not significantly decrease compared to SPIONs alone even at high concentrations of nanoparticles. The mechanisms underlying radiosensitization are still incompletely understood and more studies are needed to be conducted with this regards.
Conclusion
This study revealed that PG-SPIONs are not cytotoxic on U87-MG cancer cells even at concentrations up to 200 µg/ml. Results of MTT test showed that cell viability decreases significantly by increasing radiation doses. Based on our result, we can conclude that PG-SPIONs combination with 6 MV X-ray reduced survival of U87-MG cells, it is not a good radiosensitizer to be used for radiotherapy.
Acknowledgement
We thank Hamadan and Isfahan Universities of medical sciences and biotechnology laboratory of Isfahan University for their kindly supports.
Conflict of Interest: None
References
- 1.Huang G, Chen H, Dong Y, Luo X, Yu H, Moore Z, et al. Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics. 2013;3:116–26. doi: 10.7150/thno.5411. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babaei M, Ganjalikhani M. The potential effectiveness of nanoparticles as radio sensitizers for radiotherapy. Bioimpacts. 2014;4:15–20. doi: 10.5681/bi.2014.003. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoei S, Mahdavi S R, Fakhimikabir H, Shakeri-Zadeh A, Hashemian A. The role of iron oxide nanoparticles in the radiosensitization of human prostate carcinoma cell line DU145 at megavoltage radiation energies. Int J Radiat Biol. 2014;90:351–6. doi: 10.3109/09553002.2014.888104. [DOI] [PubMed] [Google Scholar]
- 4.Su X Y, Liu P D, Wu H, Gu N. Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol Med. 2014;11:86–91. doi: 10.7497/j.issn.2095-3941.2014.02.003. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Retif P, Pinel S, Toussaint M, Frochot C, Chouikrat R, Bastogne T, et al. Nanoparticles for Radiation Therapy Enhancement: the Key Parameters. Theranostics. 2015;5:1030–44. doi: 10.7150/thno.11642. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haume K, Rosa S, Grellet S, Smialek M A, Butterworth K T, Solov’yov A V, et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol. 2016;7:8. doi: 10.1186/s12645-016-0021-x. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney G P, Barton M B. Evidence-based estimates of the demand for radiotherapy. Clin Oncol (R Coll Radiol) 2015;27:70–6. doi: 10.1016/j.clon.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363–72. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 9.Amelio D, Amichetti M. Radiation therapy for the treatment of recurrent glioblastoma: an overview. Cancers (Basel) 2012;4:257–80. doi: 10.3390/cancers4010257. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raizer J, Parsa A. Current understanding and treatment of gliomas: Springer; 2015. [Google Scholar]
- 11.Huynh G H, Deen D F, Szoka Jr F C. Barriers to carrier mediated drug and gene delivery to brain tumors. J Control Release. 2006;110:236–59. doi: 10.1016/j.jconrel.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Invernici G, Cristini S, Alessandri G, Navone S E, Canzi L, Tavian D, et al. Nanotechnology advances in brain tumors: the state of the art. Recent Pat Anticancer Drug Discov. 2011;6:58–69. doi: 10.2174/157489211793979990. [DOI] [PubMed] [Google Scholar]
- 13.Rozhkova E A. Nanoscale materials for tackling brain cancer: recent progress and outlook. Adv Mater. 2011;23:H136–50. doi: 10.1002/adma.201004714. [DOI] [PubMed] [Google Scholar]
- 14.Ling Y, Wei K, Zou F, Zhong S. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Int J Pharm. 2012;430:266–75. doi: 10.1016/j.ijpharm.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Wen P Y, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 16.Ostrom Q T, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16 Suppl 4:iv1–63. doi: 10.1093/neuonc/nou223. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang C, Wang K, Stephen Z R, Mu Q, Kievit FM, Chiu D T, et al. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl Mater Interfaces. 2015;7:6674–82. doi: 10.1021/am5092165. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker M D, Strike T A, Sheline G E. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–31. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 19.Ruben J D, Dally M, Bailey M, Smith R, McLean C A, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Lu A H, Salabas E L, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46:1222–44. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 21.Kanwar J R, Mahidhara G, Kanwar R K. Recent advances in nanoneurology for drug delivery to the brain. Current nanoscience. 2009;5:441–8. [Google Scholar]
- 22.Pankhurst Q A, Connolly J, Jones S, Dobson J. Applications of magnetic nanoparticles in biomedicine. Journal of physics D: Applied physics. 2003;36:R167. [Google Scholar]
- 23.Chertok B, Moffat B A, David A E, Yu F, Bergemann C, Ross B D, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–96. doi: 10.1016/j.biomaterials.2007.08.050. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjipanayis C G, Machaidze R, Kaluzova M, Wang L, Schuette A J, Chen H, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–12. doi: 10.1158/0008-5472.CAN-10-1022. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun S, Oppermann H, Mueller A, Renner C, Hovhannisyan A, Baran-Schmidt R, et al. Hedgehog signaling in glioblastoma multiforme. Cancer Biol Ther. 2012;13:487–95. doi: 10.4161/cbt.19591. [DOI] [PubMed] [Google Scholar]
- 26.Huang F K, Chen W C, Lai S F, Liu C J, Wang C L, Wang C H, et al. Enhancement of irradiation effects on cancer cells by cross-linked dextran-coated iron oxide (CLIO) nanoparticles. Phys Med Biol. 2010;55:469–82. doi: 10.1088/0031-9155/55/2/009. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Sommer A, Distel L V, Neuhuber W, Kryschi C. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem Biophys Res Commun. 2012;425:393–7. doi: 10.1016/j.bbrc.2012.07.108. [DOI] [PubMed] [Google Scholar]
- 28.Roeske J C, Nunez L, Hoggarth M, Labay E, Weichselbaum R R. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol Cancer Res Treat. 2007;6:395–401. doi: 10.1177/153303460700600504. [DOI] [PubMed] [Google Scholar]
- 29.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–8. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 30.Hu F, Neoh K G, Cen L, Kang E T. Cellular response to magnetic nanoparticles “PEGylated” via surface-initiated atom transfer radical polymerization. Biomacromolecules. 2006;7:809–16. doi: 10.1021/bm050870e. [DOI] [PubMed] [Google Scholar]
- 31.Lutz J F, Stiller S, Hoth A, Kaufner L, Pison U, Cartier R. One-pot synthesis of pegylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules. 2006;7:3132–8. doi: 10.1021/bm0607527. [DOI] [PubMed] [Google Scholar]
- 32.Frey H. Hyperbranched polyglycerols (Synthesis and Applications) Encyclopedia of Polymeric Nanomaterials. 2015:977–80. [Google Scholar]
- 33.Saucier-Sawyer J K, Deng Y, Seo Y E, Cheng C J, Zhang J, Quijano E, et al. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J Drug Target. 2015;23:736–49. doi: 10.3109/1061186X.2015.1065833. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Saucier-Sawyer J K, Hoimes C J, Zhang J, Seo Y E, Andrejecsk J W, et al. The effect of hyperbranched polyglycerol coatings on drug delivery using degradable polymer nanoparticles. Biomaterials. 2014;35:6595–602. doi: 10.1016/j.biomaterials.2014.04.038. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maity D, Choo S-G, Yi J, Ding J, Xue J M. Synthesis of magnetite nanoparticles via a solvent-free thermal decomposition route. Journal of Magnetism and Magnetic Materials. 2009;321:1256–9. [Google Scholar]
- 36.Zhao L, Chano T, Morikawa S, Saito Y, Shiino A, Shimizu S, et al. Hyperbranched polyglycerol-grafted superparamagnetic iron oxide nanoparticles: synthesis, characterization, functionalization, size separation, magnetic properties and biological applications. Advanced Functional Materials. 2012;22:5107–17. [Google Scholar]
- 37.Indira T, Lakshmi P. Magnetic nanoparticles—a review. Int J Pharm Sci Nanotechnol. 2010;3:1035–42. [Google Scholar]
- 38.Mody V V, Cox A, Shah S, Singh A, Bevins W, Parihar H. Magnetic nanoparticle drug delivery systems for targeting tumor. Applied Nanoscience. 2014;4:385–92. [Google Scholar]
- 39.Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharmaceutica Sinica B. 2013;3:65–75. [Google Scholar]
- 40.Kratzke R A, Kramer B S. Evaluation of in vitro chemosensitivity using human lung cancer cell lines. J Cell Biochem Suppl. 1996;24:160–4. doi: 10.1002/jcb.240630511. [DOI] [PubMed] [Google Scholar]
- 41.Franken N A, Rodermond H M, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 42.Buch K, Peters T, Nawroth T, Sänger M, Schmidberger H, Langguth P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay-A comparative study. Radiation oncology. 2012;7:1. doi: 10.1186/1748-717X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou T, Martin N. CompuSyn software for drug combinations and for general dose-effect analysis and user’s guide. Paramus : ComboSyn Inc; 2007. [Google Scholar]
- 44.Tartaj P, Serna C J. Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites. J Am Chem Soc. 2003;125:15754–5. doi: 10.1021/ja0380594. [DOI] [PubMed] [Google Scholar]
- 45.Marinin A. Synthesis and characterization of superparamagnetic iron oxide nanoparticles coated with silica. School of Information and Communication Technology Royal Institute of Technology: Stockholm; 2012. [Google Scholar]
- 46.Keshavarzi E, Ghaeb Y, Rouhani S F. The magnetic properties of Fe3O4 nanoparticale with different Coats and hydrodynamic diameters. c2010. Avilable From: [http://www.civilica.com/Paper-ISPTC12-ISPTC12_121.html. ]
- 47.Wang L, Neoh K, Kang E, Shuter B, Wang S C. Superparamagnetic hyperbranched polyglycerol-grafted Fe3O4 nanoparticles as a novel magnetic resonance imaging contrast agent: an in vitro assessment. Advanced Functional Materials. 2009;19:2615–22. [Google Scholar]
- 48.Ankamwar B, Lai T-C, Huang J-H, Liu R-S, Hsiao M, Chen C-H, et al. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology. 2010;21:075102. doi: 10.1088/0957-4484/21/7/075102. [DOI] [PubMed] [Google Scholar]
- 49.Choi J Y, Lee S H, Na H B, An K, Hyeon T, Seo T S. In vitro cytotoxicity screening of water-dispersible metal oxide nanoparticles in human cell lines. Bioprocess Biosyst Eng. 2010;33:21–30. doi: 10.1007/s00449-009-0354-5. [DOI] [PubMed] [Google Scholar]
- 50.Mahmoudi M, Hofmann H, Rothen-Rutishauser B, Petri-Fink A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev. 2012;112:2323–38. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- 51.Harvey K A, Xu Z, Saaddatzadeh M R, Wang H, Pollok K, Cohen-Gadol A A, et al. Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines. J Neurosurg. 2015;122:547–56. doi: 10.3171/2014.10.JNS14759. [DOI] [PubMed] [Google Scholar]
- 52.Sutradhar K B, Amin M L. Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnology. 2014;2014 [Google Scholar]
- 53.Saatchi K, Gelder N, Gershkovich P, Sivak O, Wasan K M, Kainthan R K, et al. Long-circulating non-toxic blood pool imaging agent based on hyperbranched polyglycerols. Int J Pharm. 2012;422:418–27. doi: 10.1016/j.ijpharm.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Tubiana M. Introduction to radiobiology. Florida : CRC Press; 2005. [Google Scholar]
- 55.Hall E J, Giaccia A J. Radiobiology for the Radiologist. Philadelphia : Lippincott Williams & Wilkins ; 2006. [Google Scholar]
- 56.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 57.Jia H Y, Liu Y, Zhang X J, Han L, Du L B, Tian Q, et al. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J Am Chem Soc. 2009;131:40–1. doi: 10.1021/ja808033w. [DOI] [PubMed] [Google Scholar]
- 58.Klein S, Dell’Arciprete M L, Wegmann M, Distel L V, Neuhuber W, Gonzalez M C, et al. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem Biophys Res Commun. 2013;434:217–22. doi: 10.1016/j.bbrc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 59.Misawa M, Takahashi J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomedicine. 2011;7:604–14. doi: 10.1016/j.nano.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Gara P M D, Garabano N I, Portoles M J L, Moreno M S, Dodat D, Casas O R, et al. ROS enhancement by silicon nanoparticles in X-ray irradiated aqueous suspensions and in glioma C6 cells. J Nanopart Res. 2012;14:741. [Google Scholar]
- 61.Lehnert S. Radiosensitizers and Radiochemotherapy in the Treatment of Cancer. New York : CRC Press; 2014. [Google Scholar]