Abstract
Silk fibroin is a biomaterial with multiple beneficial properties for use in regenerative medicine and tissue engineering. When dissolving and processing the reconstituted silk fibroin solution by electrospinning, the arrangement and size of fibers can be manifold varied and according fiber diameters reduced to the nanometer range. Such nonwovens show high porosity as well as potential biocompatibility. Usually, electrospinning of most biomaterials demands for the application of additives, which enable stable electrospinning by adjusting viscosity, and are intended to evaporate during processing or to be washed out afterwards. However, the use of such additives increases costs and has to be taken into account in terms of biological risks when used for biomedical applications.
In this study, we explored the possibilities of additive-free electrospinning of pure fibroin nonwovens and tried to optimize process parameters to enable stable processing. We used natural silk derived from the mulberry silkworm Bombyx mori. After degumming, the silk fibroin was dissolved and the viscosity of the spinning solution was controlled by partial evaporation of the initial solving agent. This way, we were able to completely avoid the use of additives and manufacture nonwovens, which potentially offer higher biocompatibility and reduced immunogenicity. Temperature and relative humidity during electrospinning were systematically varied (25–35 °C, 25–30% RH). In a second step, the nonwovens optionally underwent methanol treatment to initiate beta-sheet formation in order to increase structural integrity and strength. Comprehensive surface analysis on the different nonwovens was performed using scanning electron microscopy and supplemented by additional mechanical testing. Cytotoxicity was evaluated using BrdU-assay, XTT-assay, LDH-assay and live-dead staining. Our findings were, that an increase of temperature and relative humidity led to unequal fiber diameters and defective nonwovens. Resistance to penetration decreased accordingly. The most uniform fiber diameters of 998 ± 63 nm were obtained at 30 °C and 25% relative humidity, also showing the highest value for resistance to penetration (0.20 N). The according pure fibroin nonwoven also showed no signs of cytotoxicity. However, while the biological response showed statistical evidence, the material characteristics showed no statistically significant correlation to changes of the ambient conditions within the investigated ranges. We suggest that further experiments should explore additional ranges for temperature and humidity and further focus on the repeatability of material properties in dependency of suitable process windows.
Keywords: Fibroin, Additive-free electrospinning, Nanofiber, Silk, Scaffolds
Graphical abstract
Highlights
-
•
Usually, electrospinning of most biomaterials demands for the application of additives. However, the use of such additives increases costs and has to be taken into account in terms of biological risks.
-
•
After degumming, fibroin was dissolved and the viscosity of the spinning solution was controlled by partial evaporation of the initial solving agent. In this way, we were able to completely avoid the use of additives.
-
•
Using a pure fibroin solution contributes to higher biocompatibility and reduces immunogenicity of the products.
-
•
Increase of temperature and humidity led to unequal fiber diameters and defective nonwovens. The most uniform fiber diameters of 998 ± 63 nm were obtained at 30 °C and 25% RH.
1. Introduction
Silk is a biomaterial which shows several advantages over synthetic or natural polymers in tissue engineering. High biocompatibility, mechanic stability, controllable degradation and high porosity may favor the use of silk as scaffolds [1,2], e.g. for regeneration of tissue defects [[3], [4], [5], [6], [7], [8], [9], [10]], replacement of organs [[11], [12], [13], [14]] or for drug-delivery-systems [15]. Silk derived products can be combined with anticoagulant drugs to improve hemocompatibility [16] as well as antibacterial agents [17] or stem cells [18] to support wound healing.
Originally secreted by the silkworm Bombyx mori to form cocoons for metamorphosis, silk fibers consist mostly of the protein fibroin, which are bundled by the protein sericin [[19], [20], [21]]. A strong inflammation reaction may occur when raw silk fibers comprising both, fibroin and sericin at the same time are used in humans. Hence, for biomedical applications degumming in cleaning solutions is used to wash out and minimize the content of sericin. After degumming of the raw silk, the purified fibroin can be used among others to build nonwovens using electrospinning [22]. The resulting fibers are characterized by a high specific surface, porosity and heat resistance up to 250 °C, which may be important in order to be processed by thermal sterilization techniques [20]. When used in vivo, minimal immune response during degradation can be observed for nonwovens consisting of pure fibroin [23,24]. Amongst others, electrical conductivity and viscosity of the spinning solution are critical parameters for a stable spinning process [22,25]. These parameters can be controlled with additives such as polyethylene oxide [1]. However, these substances may remain in the finished product and may have adverse effects on biocompatibility or with regard to immunogenicity. Therefore, our aim was to determine suitable process parameters for the stable electrospinning of fibroin nonwovens, while waiving the use of typical electrospinning additives, and to determine their morphological and mechanical properties for potential use in biomedical applications.
2. Methods
2.1. Preparation of the fibroin solution
All silk solutions used for electrospinning were provided by Fibrothelium GmbH, Aachen, Germany. Briefly, silk cocoons of the mulberry silkworm (Bombyx mori) were sourced locally in the European Union, hackled and boiled in 0,05 mol-% sodium carbonate solution (Na2CO3) for 1 h in order to remove sericin. After drying, the degummed silk fibroin wool was dissolved and purified in a proprietary process using a solution based on Ajisawa's reagent at 80 °C for 1 h and customized equipment for purification. After eliminating all contaminations and dirt originating from the cocoons, the solution was dialyzed by means of a semipermeable membrane against distilled water for at least 24 h. Afterwards the concentration of the fibroin spinning solution was measured to be 4 to 5 wt-%. Polyethylene oxide (PEO, Sigma Aldrich Chemie GmbH, Taufkirchen, Germany) was added to one fraction of the fibroin solution (ratio 1:4 and 1:8), while the remainder maintained free of additives. An increase of viscosity in the additive-free fraction of the fibroin solution was accomplished by partial evaporation of the solvent at 60–70 °C under stirring (200 RPM) for different time periods.
2.2. Electrospinning of the fibroin solution
PEO containing as well as additive-free fibroin solutions were spun at the Institut für Textiltechnik of RWTH Aachen University (ITA), Aachen, Germany, using commercial electrospinning equipment (Fluidnatek LE-500, BIOINICIA SL, Paterna, Spain) and a single spinneret setup. Plain and polished metal sheets of 0.1 mm stainless steel (M-Tech, Georg Martin GmbH, Dietzenbach, Germany) were used as top layer for the collector. For all trials a stainless-steel cannula with 0.6 mm inner diameter and 0.8 mm outer diameter was used as spinneret. To ensure constant feeding of the spinning solution, a 5 ml syringe was used with a commercially available syringe pump (B. Braun FM Perfusor, B. Braun Deutschland GmbH & Co. KG, Melsungen, Germany). Distance from cannula tip to the collector was fixed at 180 mm. Process parameters being varied, such as applied voltage, flow rate, fibroin concentration, additives, temperature and relative humidity are being presented in the results section.
2.3. Post-treatment of the pure fibroin nonwovens
Post-treatment of the fibroin nonwovens to induce beta-sheet formation was achieved by immersion in 90% (v/v) aqueous methanol solution (CH4O) for 20 min.
2.4. Morphological and mechanical evaluation
2.4.1. Electrical conductivity of the spinning solution
Electric conductivity of the spinning solution was evaluated using a conductivity meter (GLF 100, GHM Messtechnik GmbH, Regenstauf, Germany) measuring ion conductivity in the surrounding medium. To obtain stable measurements the graphite electrode was immersed at least 30 mm deep and allowed to rest for at least 1 min.
2.4.2. Dynamic viscosity of the spinning solution
Viscosity was measured in a rotary viscosimeter (RheolabQC, Anton Paar GmbH, Graz, Austria). Using testing cup CC10, 0.97 ml of spinning solution was analyzed with varying shear rates of 0–1500 s−1 and dynamic viscosity was calculated.
2.4.3. Scanning electron microscopy of the fibroin nonwovens
A scanning electron microscope (SEM, XL30 SEM, FEI Europe B.V., Eindhoven, Netherlands) was used to examine the surface morphology of the fibers. The non-conducting specimens were coated with a flash of gold using a sputter coater (S150B, Edward Ltd., Crawley, England) in order to assess the fiber cross-section as well as the structure and porosity of the nonwovens. The average fiber diameter of the nonwovens was assessed using Fiji open source Java image processing (Laboratory for Optical and Computational Instrumentation (LOCI) of the University of Wisconsin-Madison, Madison, USA). Average fiber diameter and standard deviation were calculated with a sample size of n = 10.
2.4.4. Thickness measurement of the fibroin nonwovens
The thickness of the nonwovens was assessed using a micrometer screw (Micromar 40 ER, Mahr GmbH, Göttingen, Germany). Maximum thickness was determined as maximum distance between the peak points of the upper and lower interface between the nonwovens and the plain contact area of the micrometer screw.
2.4.5. Mechanical resistance of the fibroin nonwovens
The mechanical resistance of the nonwovens to penetration was assessed using a material testing machine (Zwick/Roell Z010 XForceK, Zwick GmbH & Co. KG, Ulm, Germany) and a customized setup for penetration testing according to DIN EN 14477 [26]. Round specimens of the nonwovens with diameter of 10 mm or bigger were fixed by a support ring and a sharp rounded punch was lowered with a speed of 5 mm/min until the specimens were fully penetrated.
Statistical analysis facilitating Pareto analysis was performed to test for significance levels of temperature and relative humidity (confidence level of 90%).
2.5. Biological evaluation (cytotoxicity)
2.5.1. Sterilization of specimens
All samples (nonwovens and reference materials) were sterilized by immersion in isopropanol for 5 min with subsequent drying in a laminar flow hood.
2.5.2. Reference materials
RM-A, a polyurethane film containing 0.1% zinc diethyldithiocarbamate (ZDEC) that was obtained from the Hatano Research Institute, Food and Drug Safety Center, Japan, was used as reference material for positive control. For the live-dead staining assay, Tissue Culture Coverslips (TCC) (Sarstedt AG & Co. KG, Nümbrecht, Germany, Cat. No. 83.1840.002) were used as nontoxic reference material for negative control. Samples of RM-A and TCC were used with the identical surface areas as the material specimens tested.
2.5.3. Cell culture
L-929 mouse fibroblasts were obtained from the European Collection of Cell Culture, ECACC, Salisbury, UK. Cells were cultured in MEM (Minimum Essential Medium) supplemented with 10% fetal bovine serum, penicillin/streptomycin (100 U/mL each) (all from Life Technologies Corp., Carlsbad, USA) and l-glutamine (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) to a final concentration of 4 mM (in the following referred to as “cell culture medium”) at 37 °C, 5% CO2 and 95% RH (referred to as “cell culture conditions”). Cells were passaged when they reached about 80% confluency.
2.5.4. Indirect cytotoxicity testing
For indirect cell culture testing, specimens (n = 3) and positive control samples were extracted with 3 cm2/ml of cell culture medium for 72 h under cell culture conditions. Cell culture medium only was incubated under identical conditions to serve as a negative control extract. After removal of the specimens, the remaining extracts were centrifuged at 14,000 RPM for 10 min. The supernatants were subsequently used for the assay procedure: 96 well plates were seeded with 1 × 104 L929 cells/well in 100 μl cell culture medium and incubated under cell culture conditions for 24 h. Thereafter, cell culture medium was discarded and 100 μl of extract were added to each well. Cells were further incubated for 24 h and then subjected to the BrdU- and XTT-assays while the supernatants were subjected to the LDH-assay. Absorbance values of blank controls (only medium without cells) were subtracted from the assay values to adjust the readout. For the BrdU-assay, the Cell Proliferation ELISA, BrdU (colorimetric) test kit (Roche Diagnostics GmbH, Mannheim, Germany) was used according to the manufacturer's instructions. Briefly, cells were labeled with BrdU for 2 h under cell culture conditions and subsequently fixed for 30 min at room temperature (RT) with FixDenat reagent. Then, the fixed cells were incubated for 1 h with anti-BrdU-POD antibody and washed 3 times for 5 min with washing buffer. The immune complexes were detected after a subsequent substrate reaction with tetramethyl-benzidine (TMB) (20 min at room temperature) followed by addition of 25 μl 1 M H2SO4 to stop the reaction using a scanning multi-well spectrophotometer (ELISA reader) with filters for 450 nm and 690 nm (reference wavelength). For the XTT-assay, the Cell Proliferation Kit II (XTT) (Roche Diagnostics GmbH, Mannheim, Germany) was used according to the manufacturer's instructions. Briefly, the electron-coupling reagent was mixed with XTT labeling reagent (1:50 dilution) and 50 μl of the mixture was added to the cells. After 4 h of incubation under cell culture conditions, substrate conversion was quantified by measuring the absorbance of 100 μl aliquots in a new 96 well plate using a scanning multi-well spectrophotometer (ELISA reader) with filters for 450 nm and 650 nm (reference wavelength). For the XTT-assay, the Cell Proliferation Kit II (XTT) (Roche Diagnostics GmbH, Mannheim, Germany) was used according to the manufacturer's instructions. Briefly, the electron-coupling reagent was mixed with XTT labeling reagent (1:50 dilution) and 50 μl of the mixture was added to the cells. After 4 h of incubation under cell culture conditions, substrate conversion was quantified by measuring the absorbance of 100 μl aliquots in a new 96 well plate using a scanning multi-well spectrophotometer (ELISA reader) with filters for 450 nm and 650 nm (reference wavelength). For the LDH-assay, the LDH-Cytotoxicity Assay Kit II (BioVision, Milpitas, CA 95035, USA) was used according to the manufacturer's instructions. Briefly, 10 μl of the cell supernatants were incubated with 100 μl LDH reaction mix for 30 min at room temperature. After addition of stopping solution, absorbances were measured using a scanning multi-well spectrophotometer (ELISA reader) with filters for 450 nm and 650 nm (reference wavelength).
2.5.5. Direct cytotoxicity testing
For direct cell culture testing, specimens and reference materials were seeded with 2.4 × 105 cells in 1 ml cell culture medium in 12 well plates. Cells were seeded directly onto the surface of the materials. Assays were carried out after 24 h incubation under cell culture conditions. In order to perform live-dead cell staining on the surfaces of the specimens, 60 μl per ml medium propidium iodide (PI) stock solution (50 μg/ml in PBS) and 500 μl per ml medium fresh fluorescein diacetate (FDA) working solution (20 μg/ml in PBS from 5 mg/ml FDA in acetone stock solution) were added to each well (12 well plate). After a brief incubation for 3 min at room temperature, specimens were rinsed in prewarmed PBS and were immediately examined with a fluorescence microscope equipped with a filter for parallel detection of red and green fluorescence.
For all biological tests, statistical analysis facilitating paired T-tests were performed to test for significance levels of temperature and relative humidity (confidence level of 95%).
3. Results
3.1. PEO as additive for the electrospinning of silk fibroin
In the beginning of our study, preliminary experiments on the role and necessity of additives for the electrospinning of fibroin were conducted. PEO might be considered one of the most common additives to enable electrospinning of delicate polymers such as fibroin [10]. As the original concentration of the native fibroin solution after dialysis (4–5 wt-%) is inherently too low for electrospinning, the fibroin solution was elevated to an initial concentration of 15 wt-% by partial evaporation prior to the addition of the PEO solution (5 wt-%) in two different ratios (8:1 and 4:1). While ambient conditions in the spinning cabinet were maintained at mutual levels (22 °C/30% RH), the flow rate and applied voltage were adjusted to enable or at least approach a steady Taylor cone, Table 1.
Table 1.
Process parameters of the preliminary tests including PEO as additive.
| Experiment | Fibroin concentration (wt-%) | Mixing ratio Fibroin/PEO (−) | Temperature (°C) | Relative humidity (%) | Flow rate (ml/h) | Applied voltage Spinneret (+) Collector (−) (kV) |
|---|---|---|---|---|---|---|
| A | 15 | – | 22 | 30 | 0.8 | +20/- 15 |
| B | 15 | 8:1 | 22 | 30 | 3 | +17/- 17 |
| C | 15 | 4:1 | 22 | 30 | 1.7 | +7/- 7 |
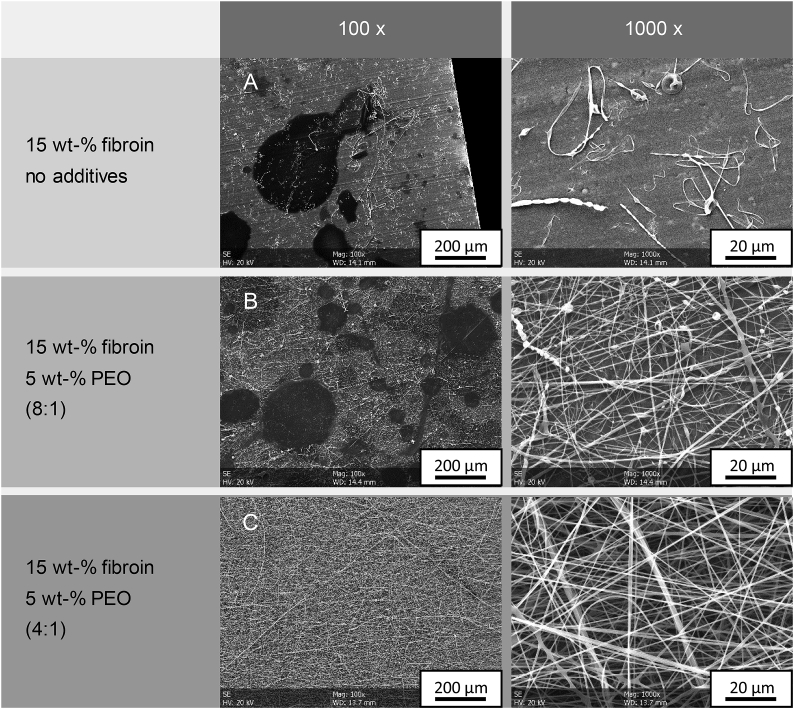
The resulting non-pure fibroin nonwovens from the preliminary tests are depicted in Fig. 1. It was shown, that with low concentration, if was not possible to create a stable Taylor cone for the electrospinning of fibroin under the used conditions (A). With the addition and increase of PEO to the initial fibroin solution, electrospinning however became increasingly feasible. While a ratio of 8:1 did still show pronounced areas of droplet formation, indicating unstable spinning conditions during processing, a further increase of PEO to a ratio of 4:1 enabled stable electrospinning of the fibroin/PEO mixture. Due to the missing or diminished integrity of the nonwovens from experiment A and B, the mean fiber diameter could only be determined on nonwoven C, amounting to 553 ± 86 nm (n = 10).
Fig. 1.
Electrospun fibroin nonwovens using PEO as additive at 20 °C/30% RH.
From the preliminary experiments, we concluded, that even using additive-free fibroin solution, initial yet unstable fiber formation could be observed during electrospinning in the facilitated setup. Furthermore, a further increase in viscosity, although induced by the addition of PEO, showed to be beneficial for the electrospinning of the additive-containing fibroin solution. However, silk fibroin and PEO did not properly blend and any mixture decomposed after only a short period of 5 min. Thus, we further focused on possibilities to increase the viscosity of pure silk fibroin solutions by means of partial evaporation.
3.2. Electrospinning of pure fibroin solutions at different concentrations
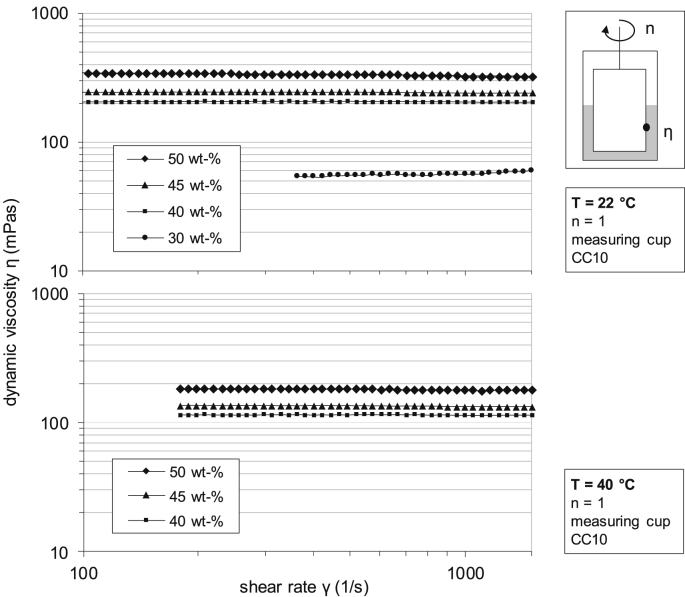
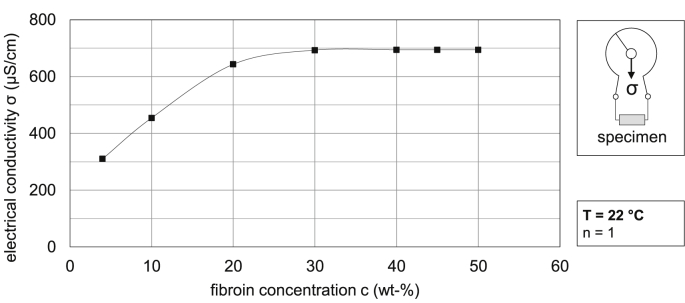
In order to evaluate electrospinning of pure fibroin at different concentrations and to relate physical parameters of the spinning solution to the spinnability, different concentrations of silk fibroin, namely 5, 10, 20, 30, 40, 45 and 50 wt-%, were prepared and measured for dynamic viscosity. Fig. 2 and Fig. 3 show the dynamic viscosity as well as the electrical conductivity of different concentrations of pure fibroin solution. Due to the explorative nature of those parameters, all measurements were single determinations.
Fig. 2.
Dynamic viscosity of different concentrations of pure fibroin solution at 22 and 40 °C.
Fig. 3.
Electrical conductivity of different concentrations of pure fibroin solution at 22 °C.
Obviously, the viscosity of the pure fibroin spinning solution drops with increasing temperature at a constant concentration level. For instance, at a fibroin concentration of 50 wt-%, the dynamic viscosity drops from 330 mPa at 22 °C to 180 mPa at 40 °C, while a decrease in dynamic viscosity from 240 mPas to 133 mPa at 45 wt-% and 205 mPas–115 mPa at 40 wt-% can be observed accordingly, Fig. 2. Due to the yet low viscosity of the solution at 30 wt-% and 22 °C (55 mPas), no valid measurements for this concentration or below could be determined at 40 °C. Even though concentrations higher than this could be measured at elevated temperature, the viscosity values were well below 200 mPas and thus, a temperature of 40 °C could generally be considered unsuitable for a stable Taylor cone formation during electrospinning, regardless of concentration. With regard to the electrical properties of the solution, Fig. 3 shows that a continuous rise in conductivity can be determined up to a fibroin concentration of 30 wt-%, when finally a threshold off app. 694 μS/cm is reached. This nonlinear behavior might result from the release and absorption of H+-ions by hydroxy or amino groups of the fibroin based amino acids. After the aqueous solution has been enriched with ions, responsible for charge transport, the conductivity reaches a limit and cannot be further increased. Hence, the increase in electrical conductivity with increasing fibroin concentration also results in more stable spinning conditions and thus further promotes the use of solutions of higher concentration, when electrospinning pure fibroin.
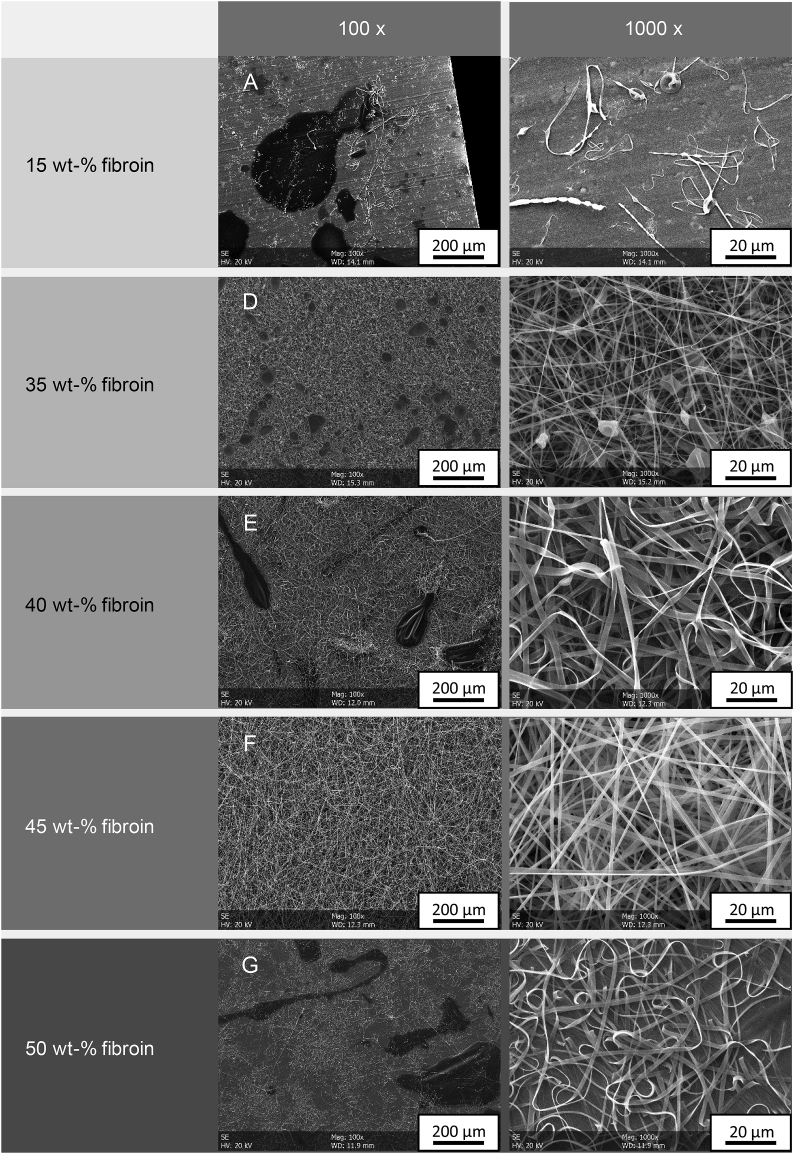
Considering the physical properties of pure fibroin solution at different concentration and temperature levels, we were able to assume inhomogeneous nonwoven formation for spinning temperatures above 40 °C and low fibroin concentrations. In order to verify or falsify this hypothesis, the previously prepared pure fibroin solutions were further subjected to our electrospinning setup and the resulting nonwovens were analyzed, Fig. 4. For process parameters, see Table 2 (A, D - G).
Fig. 4.
Nonwovens fabricated from different concentrations of pure fibroin solutions at 22 °C and 30 RH
Table 2.
Process parameters of the main experiments.
| Experiment | Fibroin concentration (wt-%) | Temperature (°C) | Relative humidity (%) | Flow rate (ml/h) | Applied voltage Spinneret (+) Collector (−) (kV) |
|---|---|---|---|---|---|
| A | 15 | 22 | 30 | 0.8 | +20/- 15 |
| D | 35 | 22 | 30 | 0.55 | +22/- 22 |
| E | 40 | 22 | 30 | 2 | +14.5/- 14.5 |
| F | 45 | 22 | 30 | 0.65 | +19/- 19 |
| G | 50 | 22 | 30 | 0.5 | +14/- 14 |
| 1 H | 45 | 25 | 25 | 0.9–1 | +20/- 20 |
| 2 I | 45 | 25 | 30 | 0.9–1 | +21/- 21 |
| 3 J | 45 | 30 | 25 | 0.9–1 | +18/- 18 |
| 4 K | 45 | 30 | 30 | 0.9–1 | +18/- 18 |
| 5 L | 45 | 35 | 25 | 0.9–1 | +18/- 18 |
| 6 M | 45 | 35 | 30 | 0.9–1 | +24/- 24 |
For fibroin concentrations at 15 wt-% (A), a stable Taylor cone and spinning jet could not be formed due to insufficient viscosity. Due to the low surface tension of the polymeric fibroin droplet at the spinneret, the continuous fiber formation is disturbed, when using these parameters. High contents of the initial solvent in the spinning solution further restrict fiber consolidation and hence only no to non-uniform nonwovens could be achieved. Facilitating higher fibroin concentrations (B/C), fiber formation was feasible but showed pronounced droplet formation. By increasing the fibroin concentration to 45 wt-% (D), uniform fibers with a diameter of 1177 ± 114 nm and drip-free nonwovens could finally be fabricated. However, a further increase in fibroin concentration (50 wt.-%) again led to insufficient fiber formation and bulging filaments due to the high dynamic viscosity of the solution. Hence, using viscosities of more than 300 mPas, a continuous flow through the spinning cannula and thus the production of drip-free nonwovens cannot be achieved. Based on these experiments, a concentration of 45 wt-% was chosen for further experiments on the dependency of the ambient conditions within the cabinet and the electrospinning of the pure silk fibroin solution.
3.3. Influence of ambient conditions on the electrospinning of pure fibroin solution
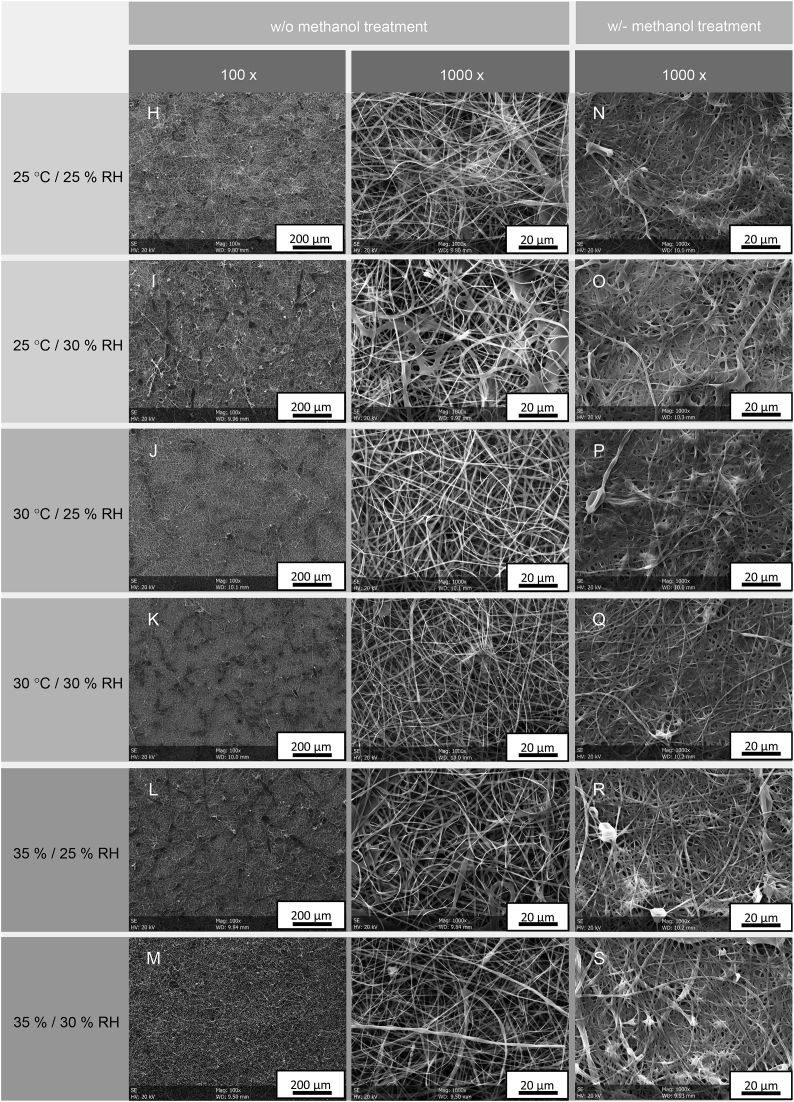
In order to determine the role of temperature and relative humidity as process parameters, a suitable process window was isolated by preliminary experiments. Briefly, 45 wt-% pure fibroin solution was electrospun at RT/40 and 50% RH and at 30 RH/25, 30 and 35 °C. The resulting nonwovens were morphologically assessed in terms of fiber diameter, homogeneity and fiber appearance (not shown). Based on these preliminary results, a set of main experiments was deduced from a full factorial experimental plan with the electrospinning temperature within the cabinet being considered the central point, Table 2 (H – M). While the temperature in the spinning cabinet was varied between 25 and 35 °C, humidity was either set to 25 or 30% RH. Resulting pure fibroin nonwovens are depicted in Fig. 5 (left).
Fig. 5.
Nonwovens fabricated from pure fibroin solutions at different temperature and relative humidity before (H–M) and after methanol treatment (N–S).
Obviously, the pure fibroin nonwovens exhibit visible differences in morphology depending on the ambient conditions within the electrospinning cabinet. While lower (25 °C) and higher temperatures (35 °C) lead to an increased defect density, e.g. droplet formation, a temperature of 30 °C seems generally well suited for the electrospinning of pure fibroin solution. In direct comparison, an operation point of 30 °C and 25% RF represented the best-case. Fig. 6 summarizes the numerical assessment of the fiber diameter derived from the morphological analysis of the nonwovens.
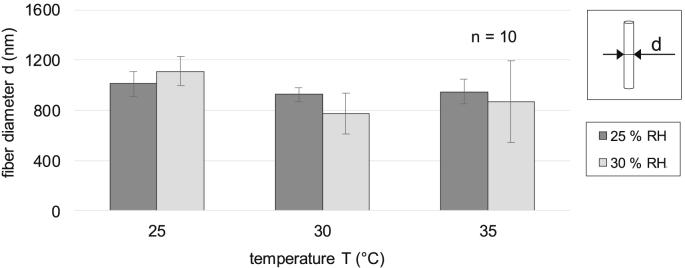
Fig. 6.
Fiber diameter of the nonwovens at different temperature and humidity.
As shown, an increase in relative humidity apparently results in higher inhomogeneity of the nonwovens, indicated by a larger standard deviation on the fiber diameter. In total, the diameter of the pure fibroin fibers in the nonwovens was around 1000 nm at average. The most homogeneous average fiber diameter of 998 ± 63 nm was obtained by electrospinning at 30 °C and 25% RH. In contrast, the largest variation in average fiber diameter, namely 418 nm, was observed for nonwovens produced at 35 °C and 30% RH. All variations showed no statistical significance due to either flat averages (e.g. 25% RH) or pronounced standard deviations (30% RH).
For further evaluation, the nonwovens were subjected to a methanol treatment. This post-treatment is a common approach to induce crystallization by beta sheet formation within the fibroin fibers and used to enable both, non-rapid degradation and mechanical stability. In our study, only by using methanol treatment it was possible to properly detach the pure fibroin nonwovens from the collector. Fig. 5 (right) shows the fiber and nonwoven morphology after post-treatment by immersion in 90% (v/v) methanol solution for 20 min. For all six fibroin nonwoven variants, methanol treatment apparently led to a reduction of porosity by densification, which in some cases also uncovered and visualized hidden droplet formation. However, it also led to occasional clogging by the treatment itself. Even though the resulting blurring in morphology prevented statistical image analyses (compare Fig. 6), a manual analysis across all groups showed that the average fiber diameter was increased by 2–20%, while the standard deviation was decreased by up to 50%. Hence, methanol treatment not only enabled mechanical stability, but also improved the homogeneity of the pure fibroin nonwovens.
To further assess the third geometrical direction of the specimens, the thickness of the different fibroin nonwovens was measured as described above. Fig. 7 shows the nonwoven thickness in dependence on the ambient conditions.
Fig. 7.
Thickness of the nonwovens at different temperatures and relative humidities.
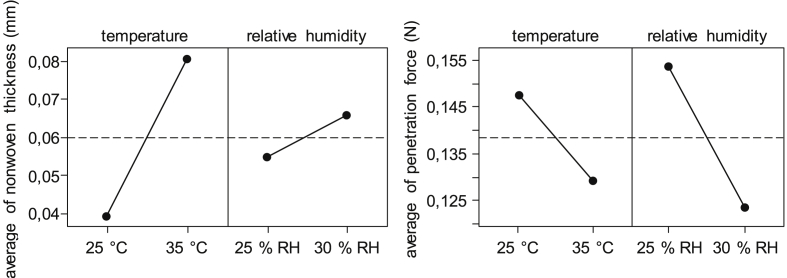
It is apparent, that at a spinning temperature of 35 °C, a pronounced standard deviation indicating an exaggerated scattering of the pure fibroin nonwoven thickness can be found regardless of the relative humidity used during electrospinning. The average thickness is higher with increasing temperature and relative humidity, e.g. from 32 to 44 μm at 30 °C and from 56 to 106 μm at 35 °C, and when relative humidity is set from 25% RH to 30% RH respectively. Only at 25 °C and 25% RH, a comparably higher thickness is measured, which cannot be correlated to a preferential cross-linking during methanol post-treatment due to insufficient differences in nonwoven morphology (compare Fig. 6). At 30 °C and 30% RH, standard deviation was omitted due to insufficient sample size (n = 1). In order to test for statistical significance a Pareto analysis was conducted, showing no significant influence of temperature or relative humidity on the nonwoven thickness at a confidence level of 90%. In addition, Fig. 8 (left) displays the effects of those parameters in a main effects plot.
Fig. 8.
Main effects plots for nonwoven thickness (left) and penetration force (right).
The effect lines show that both process parameters have main effects, while a steeper slope for temperature indicates a higher influence on the average thickness of the pure fibroin nonwovens, when compared to relative humidity.
3.4. Influence of ambient conditions on the mechanical resistance of pure fibroin nonwovens
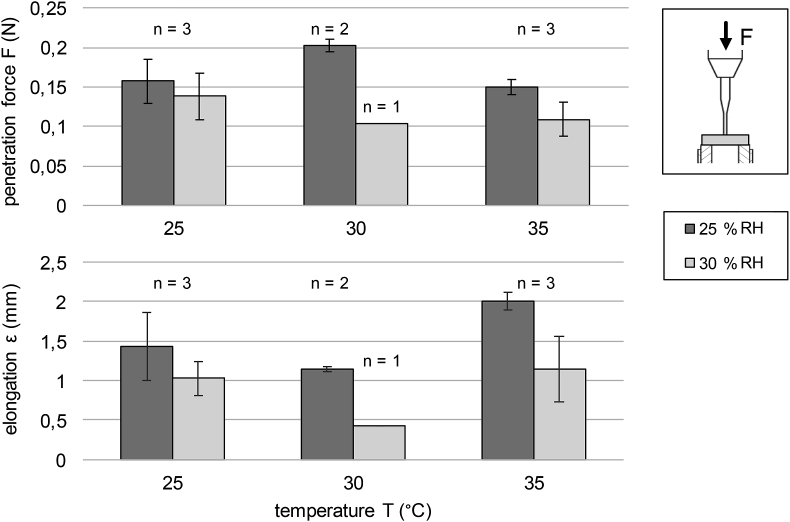
In order to test for the mechanical integrity of the nonwoven specimens in dependence of the ambient conditions, mechanical penetration tests were performed and analyzed. Fig. 9 shows the mechanical resistance to penetration and the elongation behavior in dependence of temperature and relative humidity during electrospinning.
Fig. 9.
Mechanical resistance of the nonwovens at different temperatures and relative humidities.
As can be seen in Fig. 9 (above), increasing the humidity from 25 to 30% RH generally results in a decrease of the penetration resistance, e.g. at 25 °C from 0.16 to 0.14 N, at 30 °C from 0.20 to 0.10 N and at 35 °C from 0.15 to 0.11 N. The highest resistance to penetration (0.20 N) with the lowest standard deviation (0.01 N) was observed for nonwovens spun at 30 °C and 25% RH. Both decrease of temperature to 25 °C and increase to 35 °C at 25% RH led to a decrease of resistance to perforation by app. 25%, while almost no difference could be assessed at 30% RH. Evaluation of the elongation behavior of the pure fibroin nonwovens showed no considerable differences at different temperatures and 30% RH, while at 25% RH a maximum of 2.0 mm was detected at 35 °C, Fig. 9 (below). Pareto analysis at a confidence level of 90% showed no statistically significant effect of temperature or relative humidity on the penetration resistance either. Fig. 8 (right) displays the effect lines of temperature and relative humidity on the average penetration resistance, indicating that both process parameters have main effects on the resistance average. While relative humidity shows a slightly smaller impact, both effects range in comparable orders of magnitude. Main effect analyses for the elongation properties (not shown) further showed comparably strong effects of the electrospinning temperature with contrary dependencies: While an increasing temperature elevated the elongation, an increase in relative humidity led to a diminished elongation.
In conclusion of the facilitated tests, preferred process parameters for the ambient conditions could clearly be identified. A temperature of 30 °C and 25% RH was found to be the most advantageous set ambient parameters to fabricate pure fibroin nonwovens with homogenous fiber formation, balanced thickness and high resistance to penetration. Hence, these preferred process parameters, resulting in a preferred embodiment of a pure fibroin nonwoven, were used to manufacture prototypes, which were further subjected to biological testing in order to assess their biocompatibility.
3.5. Biological evaluation of pure fibroin nonwovens
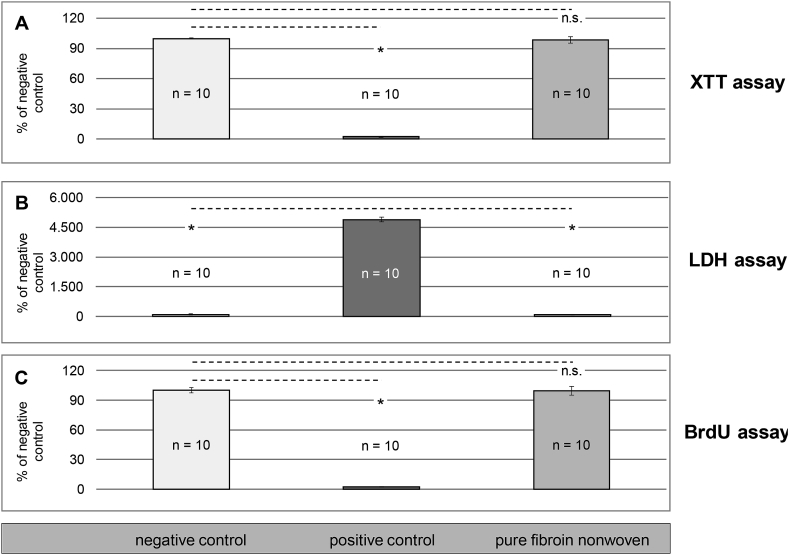
When testing biocompatibility, the international standard ISO 10993 depicts one of the most commonly consulted references [30]. Part 1 (ISO 10993-1) lists all tests to be considered in accordance with the standard, depending on the type of physical contact and the duration of contact for a medical device, e.g. a biomaterial. Cytotoxicity, referring to direct cell damage, potential influence on cell growth or specific aspects of the cell metabolism addressed by a specific toxin, is a common denominator for all types of medical devices, no matter if they are intended to be used with bone, soft tissue or in the circulatory system. Since proven toxicity in compliance with the standard can be understood as an exclusion criterion for use in humans, the basic suitability of the pure fibroin nonwovens was assessed using different methods for cytotoxicity testing. In accordance with part 5 of the standard (ISO 10993-5), indirect in-vitro assays were performed on extractive compounds of the fibroin nonwovens in contact with L929 fibroblasts, Fig. 10.
Fig. 10.
Results of indirect cytotoxicity tests on the pure fibroin nonwovens.
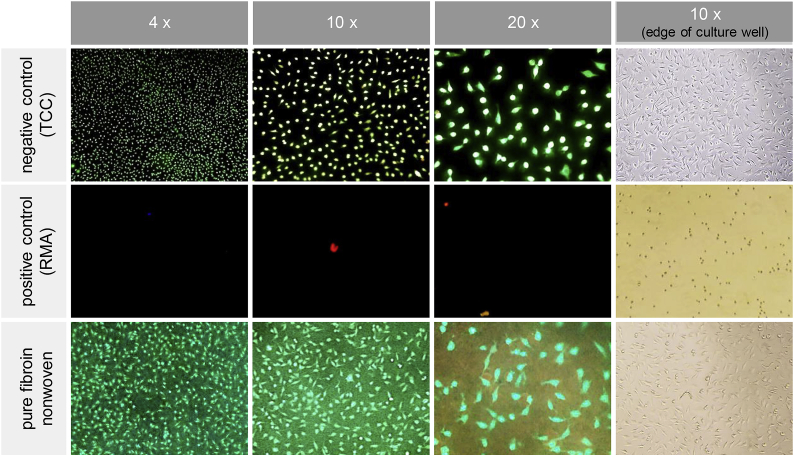
Paired student t-test with a confidence level of 99.5% (p = 0.05) was used for the XTT and BrdU tests to compare the fibroin nonwovens to the toxic positive control or non-toxic negative control by pairs. For the LDH assay, the same procedure was reversed with the positive control being the comparative basis. In brief, all cell culture assays showed statistically significant proof for the absence of cytotoxic adverse effects. The pure fibroin nonwovens consistently ranged at comparative levels of the non-toxic negative control and clearly distinguished from the toxic positive control. Mean values below 70% of the negative control (XTT and BrdU test) or above 30% of the positive control (LDH test), which are considered as cytotoxicity limits following ISO 10993-5, were far outreached. By conducting all three cell culture assays, a profound assessment of electrospun pure fibroin as a potential toxin could be performed. In conclusion, neither direct cell damage nor a reduction in metabolic condition or proliferation could be found for cells subjected to extracts of pure fibroin nonwovens. To substantiate these findings, viability and adhesion of these fibroblast cells was also assessed by direct cultivation and staining on the nonwovens and control surfaces. Three specimens (n = 3) of each, the fibroin nonwovens and both reference materials were analyzed by fluorescent reflective light microscopy and representative images were selected for display, Fig. 11.
Fig. 11.
Results of direct cytotoxicity testing (live/dead staining) of the pure fibroin nonwovens.
After live/dead staining, an almost equal amount of vital (green dyed) and well-spread spindle-shaped cells could be detected for both, pure fibroin nonwovens and the negative control (TCC). The absence of dead (red dyed) cells confirmed the assumption that no toxicity is linked with these two variants, while the toxic positive control (RMA) occasionally showed a few, but mainly no cells attached to the tested surfaces. At the edge of the culture well, validity of the conducted tests was confirmed by a reasonably dense and well-spread cell layer for the fibroin and negative control specimens, while the positive control showed an existing, yet diminished count of rounded cells.
In conclusion, both indirect and direct cytotoxicity testing indicated a good biological response of the pure fibroin nonwovens, without any signs of cytotoxic potential.
4. Discussion
Scaffolds made of pure silk fibroin may show several advantages over conventional synthetic materials. They exhibit both, high mechanical stability and degradability. Using electrospinning, fiber diameters from nanometer to micrometer scale can be achieved, facilitating high porosity and specific surface which may both promote or inhibit cell migration.
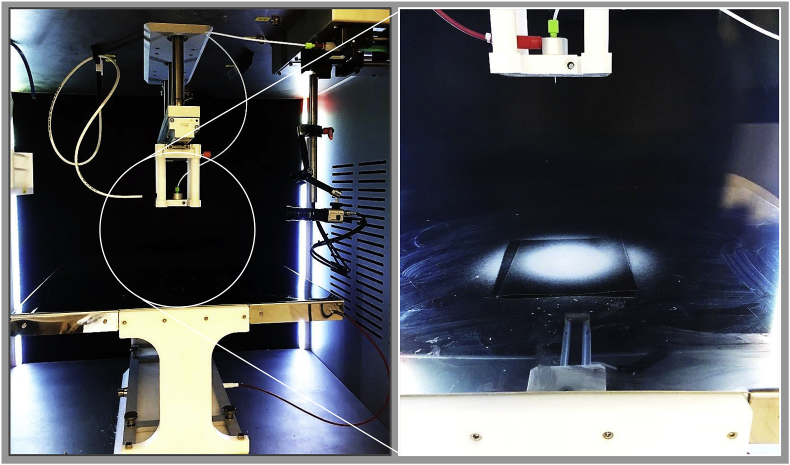
In our study, the possibilities to waive additives as PEO for the electrospinning of pure fibroin were evaluated in a first step by analyzing the physical properties of the fibroin solution. After determining appropriate viscosity levels, we were able to electrospin additive-free solution and to manufacture first pure fibroin nonwovens, Fig. 12. In a second step, we studied the influence of the ambient conditions (temperature and relative humidity) in the spinning cabinet on the electrospinning process in order to enable steady-state electrospinning of the pure fibroin solution.
Fig. 12.
Electrospinning setup (left) allowing ambient condition control and first deposition of a pure fibroin nonwoven (right).
During our preliminary experiments, we found that at temperatures above 35 °C the viscosity of the fibroin solution was too low to build up sufficient surface tension and enable a stable Taylor cone for electrospinning. Consequently, porosity as well as the deviation of the fiber diameters from the average were increased. Elevated temperatures also led to accelerated surface hardening and thus irregular fiber formation. Decelerated diffusion of the solvent through the hardened surface led to formation of a tubular polymer films on the spinning jet, further inhibiting evaporation of the solvent. In addition, higher temperatures showed to impair conditions for the storage of the fibroin solution. Bacterial growth occasionally caused degradation of the fibroin solution, resulting in reduced viscosity. Sterilization of the fibroin solution and storage at low temperatures might provide suitable strategies to prevent such contamination in future processing.
After deriving possible boundaries for the process parameters, a set of main experiments was conducted. On the basis of a systematic parameter variation, a process window for a potentially stable electrospinning process was identified. After establishing a constant concentration of 45 wt-% for the spinning solution, pure fibroin nonwovens were produced with a 0.6 mm spinning cannula at a distance of 180 mm from the collector plate. An increase in spinning temperature from 25 °C to 35 °C and relative humidity from 25% to 30% RH generally resulted in uneven fiber diameters and teardrop defects in the pure fibroin nonwovens. In addition, the penetration force was reduced with increasing temperature and relative humidity, while mechanical testing also showed that the elongation was increased. The most uniform fiber diameters of 998 ± 63 nm were determined at ambient parameters of 30 °C/25% RH, also showing the highest penetration force of 0.20 N and the lowest standard deviation of 0.03 mm from average. Accordingly, we found that the optimal spinning temperature of 30 °C was located quite central within the established boundaries for the electrospinning temperature and thus we concluded that a suitable process windows could be established. However, no statistically significant levels could be reached, neither on the dependency of the ambient conditions on morphological nor the mechanical properties of the pure fibroin nonwovens. Furthermore, main effect plots showed that no correlation was found between spinning temperature and relative humidity.
To further optimize and assess the process stability, further work should focus on studying also small variations of the process parameters, e.g. moving the spinning temperature ± 2 °C. Furthermore, additional work should aim to further push the parameter boundaries facilitated in our study, to verify or falsify additional process windows beyond the tested temperature and relative humidity ranges. While our results show that at a fibroin concentration of 45 wt-% an increase in temperature above 35 °C results in an invalid decrease in viscosity and hence leads to unstable process parameters, temperatures below 25 °C should be evaluated more into detail. Also, the effects of increasing both, fibroin concentrations and temperature could offer additional stable process windows for the electrospinning of pure fibroin solution. For example, Hodgkinson [25] reported a range of fibroin concentration between 20 and 30 wt-% for stable electrospinning and discovered the applied voltage and flow rate also to be relevant process parameters. Singh [9] used fibroin solutions of 15–21 wt-% and was able to manufacture nonwovens with fiber diameters of 183 ± 55 nm. Kishimoto [27] even reported additive-free electrospinning using a basic (pH 10–11) solution with 5 wt-% fibroin, concentration resulting from an alternative degumming process using hot water instead of alkaline reagents.
In addition to temperature and the relative humidity in the spinning cabinet (ambient conditions), the influence of the cannula diameter and the distance between the cannula and the collector should be further examined. An increase in distance between the cannula tip and the collector plate allows for higher amounts of solvent to be evaporated during polymer solidification and ultimately could enable full evaporation of the solvent without residues in the pure fibroin nonwovens.
In order to improve the mechanical properties of the pure fibroin nonwovens, the thickness or density and thus either spinning time or effective flow rate should be increased for future purposes. For example, by using coaxial nozzles with fibroin as the core polymer and an inert gas shelf, higher concentrations of fibroin and thus higher production rates could be realized, as the drying of the spinning solution at contact with air could be prevented by the gas flow in the outer nozzle. The use of coaxial tips [28] instead of regular cannulas for electrospinning or even needleless approaches might thus lead to further improvement in process efficiency.
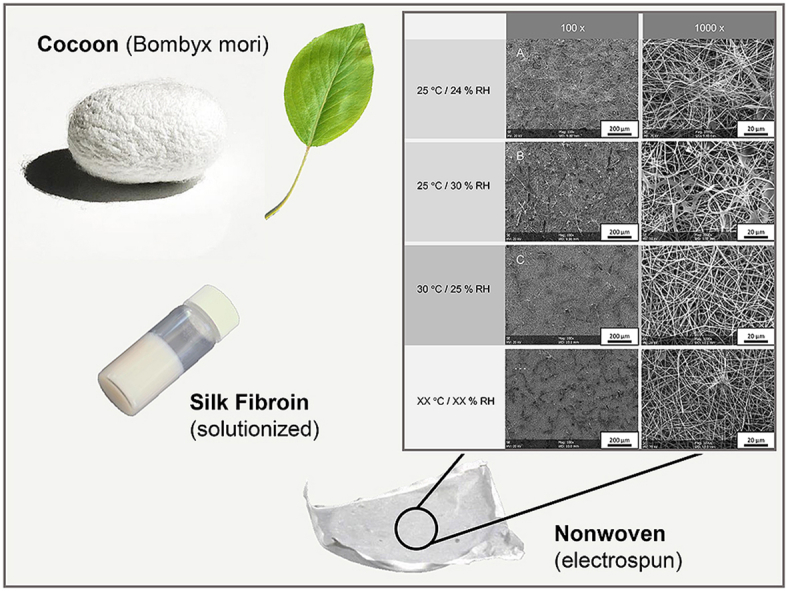
In our study, no cytotoxic effects could be detected for pure fibroin nonwovens, supporting potential use in vivo. Fig. 13 shows the evolution of the silk fibroin within our experiments. While the raw silk fibroin derived from the cocoons of Bombyx mori still showed remnants of the potentially inflammatory sericin (left), pure silk fibroin fibers were received after degumming (middle). After electrospinning of the dissolved fibroin solution without additives (right), we were able to retrieve a purified embodiment of the natural fibroin polymer, showing significantly reduced fiber diameters. Since cell-type specific morphology and high density of cells was recognized on the pure fibroin nonwovens during the life/dead stains, we hypothesize that this pure fibroin structure has high potential to be used as a bioactive material. Future work should thus evaluate, if pure fibroin is sufficient as bioactive cell carrier or if even the further addition of polymers, such as polycaprolactone, is reasonable [29]. However, the influence on the biological response needs to be tested accordingly.
Fig. 13.
Evolution of fibroin morphology within this study.
5. Conclusions
Based on the systematic variation of selected process parameters we were able to identify a suitable process window for the additive-free processing of regenerated silk fibroin by electrospinning. In this way, typical electrospinning additives as PEO could be waived. By using a 45 wt-% fibroin solution and varying spinning temperature between 25 and 35 °C as well as relative humidity between 25 and 30% RH, we found that.
-
-
an increase in spinning temperature leads to a decrease in viscosity, which encourages droplet formation,
-
-
an increase in relative humidity leads to a decrease in evaporation of the fibroin solvent and thus can lead to incomplete evaporation and fiber solidification,
-
-
the highest resistance to penetration and overall most homogenous fibroin nonwoven could be manufactured at 30 °C and 25% RH,
-
-
pure fibroin nonwovens manufactured using this process parameters showed full biocompatibility when tested for cytotoxicity
In conclusion, our results indicate that pure fibroin nonwovens can be manufactured without any additives and show promising properties for their use as potential bioactive materials in medical devices. In addition, post-treatment by methanol not only enables mechanical stability, but also improves the homogeneity of pure fibroin nonwovens.
However, further work should be done on optimizing the productivity of the electrospinning process for pure fibroin and further evaluating the biological response. With this regard, special attention should be paid on process stability, e.g. deterioration, contamination or degradation of the electrospinning solution, as well as a further variation of process parameters previously fixed in our study should be analyzed, in particular changes in the concentration of the fibroin solution, the cannula diameter of the spinneret or the distance between the spinneret and the collector.
Furthermore, the potential use of multiple spinnerets and needleless or coaxial jets should be evaluated to further improve the productivity of the process.
Ethical statement
Experimental work did not include studies on humans or animals.
CRediT authorship contribution statement
Alexander Kopp: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing. Ralf Smeets: Methodology, Resources, Supervision. Martin Gosau: Methodology, Resources. Nadja Kröger: Software, Writing - review & editing. Sandra Fuest: Validation, Visualization. Marius Köpf: Formal analysis, Supervision, Conceptualization, Resources, Supervision. Magnus Kruse: Methodology, Software, Validation, Investigation, Writing - original draft. Judith Krieger: Validation, Project administration. Rico Rutkowski: Data curation, Supervision. Anders Henningsen: Writing - original draft, Funding acquisition.
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgements
We thank Philip Hartjen and Lan Kluwe for her contribution in conducting the cytotoxicity assays as well as Igor Schestakow for his contribution in conducting the SEM analyses.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Meinel A.J. Optimization strategies for electrospun silk fibroin tissue engineering scaffolds. Biomaterials. 2009;30(17):3058–3067. doi: 10.1016/j.biomaterials.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S. Preparation, characterization and biocompatibility of electrospinning heparin-modified silk fibroin nanofibers. Int. J. Biol. Macromol. 2011;48(2):345–353. doi: 10.1016/j.ijbiomac.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Ki C.S. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: implications for bone regeneration. Biotechnol. Lett. 2008;30(3):405–410. doi: 10.1007/s10529-007-9581-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen J.P., Chen S.H., Lai G.J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res Lett. 2012;7(1):170. doi: 10.1186/1556-276X-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kundu B. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013;65(4):457–470. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2013;53:88–92. doi: 10.1016/j.ijbiomac.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Li Z. Composite poly(l-lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J. Biomater. Appl. 2016;30(10):1552–1565. doi: 10.1177/0885328216638587. [DOI] [PubMed] [Google Scholar]
- 8.Shao W. A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and Tussah silk fibroin as a scaffold for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2016;67:599–610. doi: 10.1016/j.msec.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 9.Singh B.N., Panda N.N., Pramanik K. A novel electrospinning approach to fabricate high strength aqueous silk fibroin nanofibers. Int. J. Biol. Macromol. 2016;87:201–207. doi: 10.1016/j.ijbiomac.2016.01.120. [DOI] [PubMed] [Google Scholar]
- 10.Serodio R. Ultrasound sonication prior to electrospinning tailors silk fibroin/PEO membranes for periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2019;98:969–981. doi: 10.1016/j.msec.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 11.McClure M.J. Electrospinning-aligned and random polydioxanone-polycaprolactone-silk fibroin-blended scaffolds: geometry for a vascular matrix. Biomed. Mater. 2009;4(5) doi: 10.1088/1748-6041/4/5/055010. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J., Cao C., Ma X. A novel three-dimensional tubular scaffold prepared from silk fibroin by electrospinning. Int. J. Biol. Macromol. 2009;45(5):504–510. doi: 10.1016/j.ijbiomac.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J. Electrospinning of silk fibroin and collagen for vascular tissue engineering. Int. J. Biol. Macromol. 2010;47(4):514–519. doi: 10.1016/j.ijbiomac.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Yu E. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J. Biomed. Mater. Res. 2018;106(4):985–996. doi: 10.1002/jbm.a.36297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha S.-W., Von W.E. vol. 5. Springer-Verlag Berlin; 2009. (Medizintechnik). (überarb. und erw. Aufl) [Google Scholar]
- 16.Cestari M. Preparing silk fibroin nanofibers through electrospinning: further heparin immobilization toward hemocompatibility improvement. Biomacromolecules. 2014;15(5):1762–1767. doi: 10.1021/bm500132g. [DOI] [PubMed] [Google Scholar]
- 17.Wang S.D. Improving antibacterial activity and biocompatibility of bioinspired electrospinning silk fibroin nanofibers modified by graphene oxide. ACS Omega. 2018;3(1):406–413. doi: 10.1021/acsomega.7b01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie S.Y. Adult stem cells seeded on electrospinning silk fibroin nanofiberous scaffold enhance wound repair and regeneration. J. Nanosci. Nanotechnol. 2016;16(6):5498–5505. doi: 10.1166/jnn.2016.11730. [DOI] [PubMed] [Google Scholar]
- 19.Ki C.S. The effect of residual silk sericin on the structure and mechanical property of regenerated silk filament. Int. J. Biol. Macromol. 2007;41(3):346–353. doi: 10.1016/j.ijbiomac.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y., Wang B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009;10(4):1514–1524. doi: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamboni L. Silk sericin: a versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015;33(8):1855–1867. doi: 10.1016/j.biotechadv.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Hacker C. Anlagenentwicklung für das Elektroschmelzspinnen. In: Hacker Christoph., editor. Anlagenentwicklung für das Elektroschmelzspinnen von Feinfaservliesstoffen für die Abwasseraufbereitung. - Aachen. Shaker Verlag; Aachen: 2015. Zugl. Aachen, Techn. Hochsch., Diss., 2014 u.d.T. [Google Scholar]
- 23.Wang Y. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29(24–25):3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J.H. Preparation and in vivo degradation of controlled biodegradability of electrospun silk fibroin nanofiber mats. J. Biomed. Mater. Res. 2012;100(12):3287–3295. doi: 10.1002/jbm.a.34274. [DOI] [PubMed] [Google Scholar]
- 25.Hodgkinson T. Rheology and electrospinning of regenerated bombyx mori silk fibroin aqueous solutions. Biomacromolecules. 2014;15(4):1288–1298. doi: 10.1021/bm4018319. [DOI] [PubMed] [Google Scholar]
- 26.DIN EN 14477 Bestimmung der Durchstoßfestigkeit. Prüfverfahren. Beuth Verlag GmbH; 2004. [Google Scholar]
- 27.Kishimoto Y. Electrospinning of silk fibroin from all aqueous solution at low concentration. Mater Sci Eng C Mater Biol Appl. 2017;73:498–506. doi: 10.1016/j.msec.2016.12.113. [DOI] [PubMed] [Google Scholar]
- 28.Hang Y. Preparation of regenerated silk fibroin/silk sericin fibers by coaxial electrospinning. Int. J. Biol. Macromol. 2012;51(5):980–986. doi: 10.1016/j.ijbiomac.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Roy T. Core-shell nanofibrous scaffold based on polycaprolactone-silk fibroin emulsion electrospinning for tissue engineering applications. Bioengineering. 2018;5(3) doi: 10.3390/bioengineering5030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ISO 10993 Biological Evaluation of Medical Devices. Standard. International Organization for Standardization (ISO). Geneva.