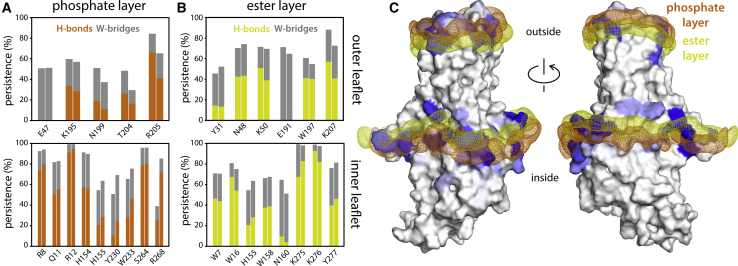
Figure 4.
Polar interactions between hDHHC20 side chains and surrounding lipids. (A) The plot quantifies the persistence of observed interactions between protein side chains and POPC phosphate groups, i.e., the fraction of the simulated time in which the interaction was detected. The data are based on two independent trajectories of 1 μs each. Two types of interactions are described: direct hydrogen bonds (H-bonds) and hydrogen bonds bridged by one water molecule (W-bridge). A direct H-bond was recorded when the distance between a protein donor and a POPC acceptor is 3.2 Å or less (POPC does not contain any H-bond donors). A W-bridge was recorded when a water molecule is found to be at 3.2 Å or less of both an H-bond acceptor in POPC and either an H-bond donor or acceptor in the protein. (B) shows the same as (A) for interactions between protein side chains and POPC ester groups. (C) Shown are 3D density maps for the phosphate and ester layers of POPC (orange and yellow mesh, respectively) near the protein surface, calculated from the MD trajectories. Protein residues that interact with either layer are colored in shades of blue; greater intensity denotes more persistent interactions, as quantified in (A) and (B). To see this figure in color, go online.