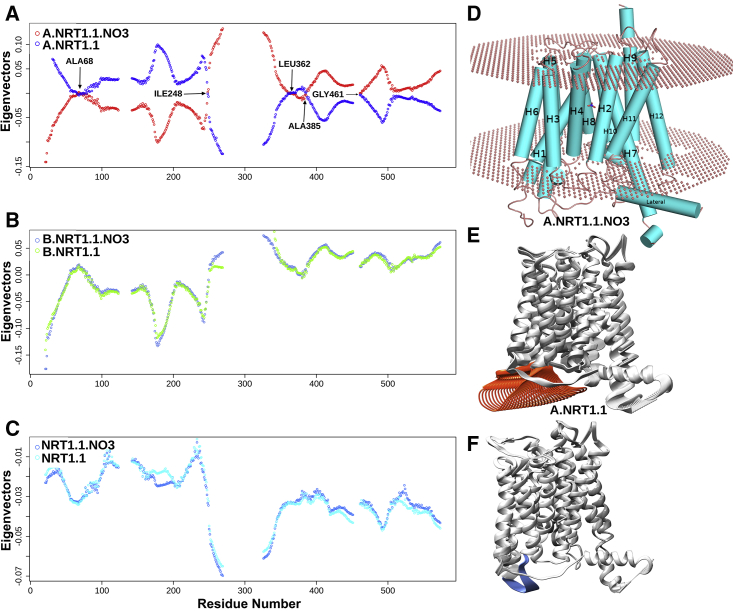
Figure 1.
Collective motion of NRT1.1 molecule shows large differences in the high-affinity state. (A) The monomeric nitrate-bound high-affinity unit shows antiphase dynamics relative to its nitrate-unbound monomer A. Several antiphase tipping residues, indicated by arrows, are responsible for maintaining this antiphase asynchronous collective motion. (B) and (C) show in-phase, synchronous motions for NRT1.1 dimer and the monomer B, with and without nitrate binding. (D) NRT1.1 monomer containing 12 transmembrane (TM)-spanning α-helices with amino-(TM1–TM6) and carboxy-terminal (TM7–TM12) bundles. (E) Nitrate-bound high-affinity monomer A shows a large-amplitude motion of the loop (orange color) connecting the C-terminal portion of helix H4 to the N-terminal of helix H5 and playing an important role for releasing the nitrate in the cytosol. (F) Without nitrate binding, such motions of the dynamic loop are absent (blue color). To see this figure in color, go online.