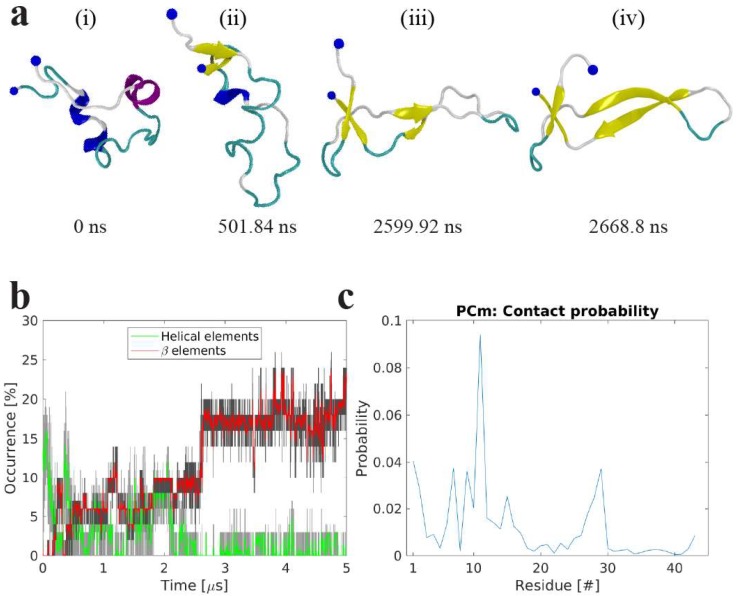
Figure 6.
Molecular dynamics simulations of the interaction of amyloid β 42 (Aβ42) monomer with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayer. (a) Snapshots showing the change of Aβ42 conformation and secondary structure at different time-points while interacting with the POPC bilayer. Protein is depicted as a cartoon following VMD coloring scheme (yellow β-strands and purple α-helices), N- and C-terminal Cα are presented as a large and a small blue sphere, respectively. (b) The evolution of secondary β-structure (β-sheet and β-bridge, red), and helical (α-, π-, and 3/10-helices, green), elements of the Aβ42 protein as determined by DSSP. The graphs are moving averages using a 1 ns window; raw data is presented as dark and light grey graphs, respectively. (c) Per residue contact probability plot between residues of the Aβ42 monomer and the P atoms of the POPC headgroups.