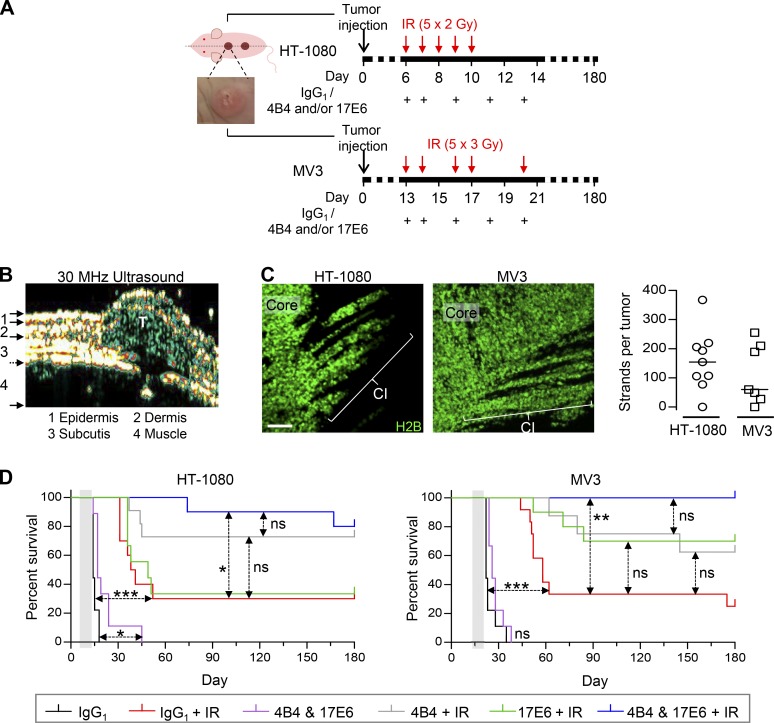
Figure 7.
Dual-targeted but not individual anti-integrin therapy to enhance radiation response, tumor eradication, and long-term survival. (A) Treatment schemes for HT-1080 and MV3 tumors. Tumor cells were injected at day 0, resulting in an intradermally growing tumor located along the dorsal midline (dashed line). Example image, intradermal HT-1080 lesion. Time points of IR and antibody administration are indicated. (B) Tumor lesion (T) after implantation in imaging window–free mouse. Intradermal localization was confirmed by high-frequency ultrasound. (C) Collective invasion (CI) pattern in intradermal tumors in imaging window–free dermis (maximum-intensity projections). Number of multicellular strands per tumor was counted from 50-µm-thick tumor sections from nine (HT-1080) and seven (MV3) tumors. Scale bar, 100 µm. (D) Tumor-free overall survival of mice after application of treatment, including fractionated IR without and with individual and dual-targeted integrin inhibition with antibodies 4B4 and/or 17E6, compared with IR combined with isotypic control antibody (representing IR alone without integrin targeting). Mice were sacrificed after 180 d or earlier, upon humane endpoint criteria (tumor size of 2 cm3, ulceration, weight loss, or poor overall condition due to internal metastasis). See Table S1 for details on mouse numbers (8–12 mice per group), metastasis formation, and tumor remnants. Gray-shaded area, therapy phase. *, P = 0.01; **, P = 0.0003; ***, P < 0.0001; ns, not significant. Statistics, log-rank survival analysis (Bonferroni-corrected thresholds: P = 0.01 [HT-1080] and P = 0.008 [MV3]).