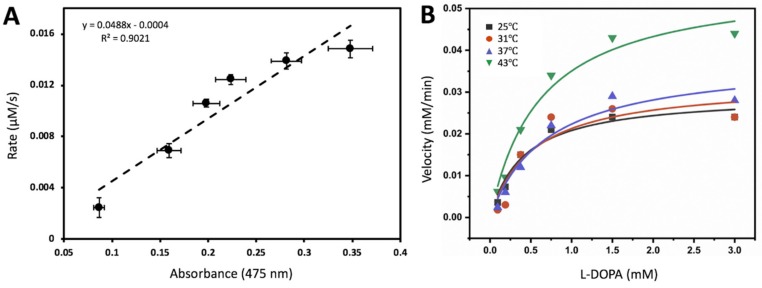
Figure 2.
Thermodynamics of tyrosinase association reaction could be recovered from temperature-dependent binding activity. (A) Isothermal titration calorimetry rate (µM/s) correlates with the dopachrome absorption. The rate of tyrosinase oxidation of L-DOPA plotted against corresponding L-DOPA concentrations as a function of absorbance values ascertained from Michaelis-Menten plot. (B) Diphenol oxidase activity of L-DOPA was measured at different temperatures. Michaelis-Menten constant and Vmax values for different temperatures is shown in Table 1. Michaelis-Menten equation y = Vmax * x/(Km + x) was used to fit data for each temperature. Temperature-dependent Tyrtr activity (averaged Vinitial and standard errors) used for the evaluation of the Michaelis-Menten constant is shown in Supplementary Material Table S1.