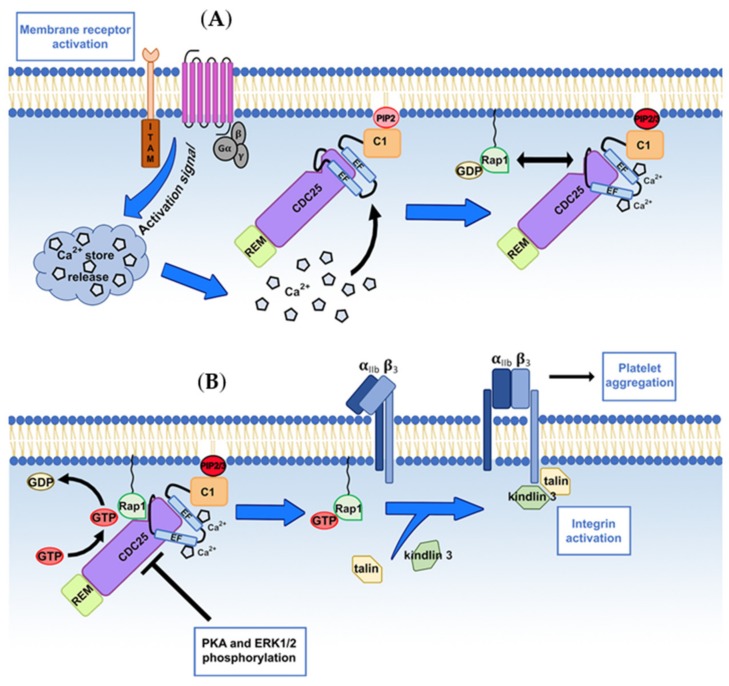
Figure 2.
RasGRP2 activation mechanism and activity regulation during αIIbβ3-integrin “inside-out” signaling in platelets. (A) Platelet surface receptor activation by vascular adhesive proteins and/or soluble agonists initiates an intracellular activation signal that induces the release of Ca2+ from intracellular stores into the platelet cytoplasm. Ca2+ binding to the EF hands induces conformational changes that activate RasGRP2, located at the platelet membrane through the association of its C1 domain with the phosphoinositides PIP2 and PIP3. (B) The membrane-bound, activated RasGRP2 interacts with Rap1 at the proximity of the cell membrane, and facilitates GDP dissociation and its replacement by (guanosine triphosphate) GTP on the GTPase. The guanine-exchange activity of RasGRP2 can be controlled by PKA- and ERK1/2-dependent phosphorylations. The GTP-bound Rap1 favors the recruitment of talin and kindlin onto the β-chain of the αIIbβ3 integrin leading to its conformational change, activation and subsequent platelet aggregation.