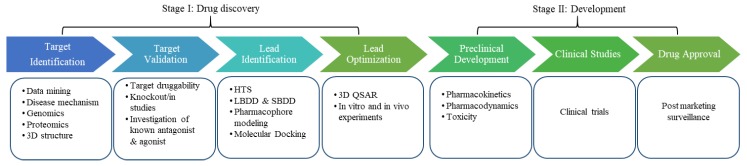
Figure 1.
The drug discovery and development pipeline. The initial step in drug discovery is target identification; the aim is to find a potential target via different approaches like genomics and proteomics. The next step is target validation, which classifies the molecular target and evaluates whether it is suitable for drug development by various in vivo and in vitro methods. Various techniques such as high-throughput screening (HTS), ligand-based drug design (LBDD), pharmacophore modeling, molecular docking, and structure-based drug design (SBDD) are employed to identify hit compounds. These hit molecules are then evaluated and modified to improve the activity of the lead compound (e.g., by quantitative SAR [QSAR] analysis). Prior to human clinical trials, preclinical studies are carried out that provide detailed knowledge on the toxicity and appropriate dose of drugs. Based on these findings, it is decided whether the drug is ready to be tested in humans. Clinical trials are the final stage of the drug development process. Once clinical trials are over, the successful drug is approved by the FDA and becomes available on the market for clinical use.