Figure 1.
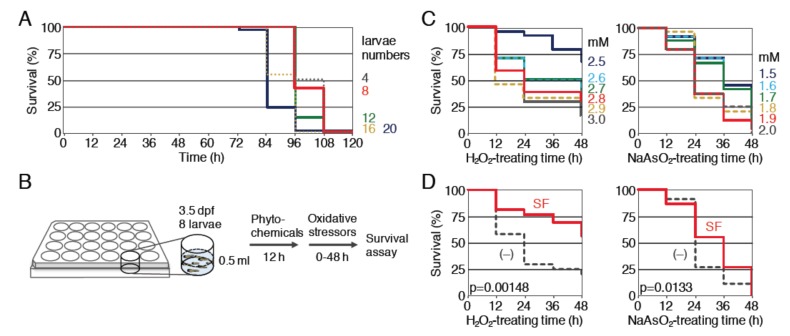
Survival assays of zebrafish larvae against oxidative stressors using 24-well plates. (A) Determination of the numbers of larvae. The indicated numbers of larvae (4 dpf) were placed into each well and survival rates were evaluated for 120 h without exchanging the medium. Larvae numbers: 4 (gray, dotted), 8 (red), 12 (green), 16 (yellow, dotted), 20 (purple). (B) A schematic diagram of the survival assay. Eight larvae (3.5 dpf) were placed into a single well of a 24-well plate, pretreated with phytochemicals for 12 h, then treated with oxidative stressors for 48 h, and the survival rates of the treated larvae were measured every 12 h. (C) Determination of the optimal concentration of oxidative stressors for the survival analysis. Larvae (4 dpf) were exposed to H2O2 at concentrations of 2.5 to 3.0 mM (left panel; 2.5 (dark blue), 2.6 (light blue, dotted), 2.7 (green), 2.8 (red), 2.9 (yellow, dotted), 3.0 mM (gray)) or NaAsO2 at concentrations of 1.5 to 2.0 mM (right panel; 1.5 (dark blue), 1.6 (light blue, dotted), 1.7 (green), 1.8 (yellow, dotted), 1.9 (red), 2.0 mM (gray, dotted)) for 48 h. Each analysis was performed in triplicate, and the experiments were repeated multiple times. (D) Antioxidant activity of sulforaphane. Larvae (3.5 dpf) were pretreated with (SF, red) or without ((–), gray, dotted) 40 µM sulforaphane for 12 h, then treated with 2.8 mM H2O2 (left panel) or 1.9 mM NaAsO2 (right panel) for 48 h, and the survival rates were analyzed every 12 h. All survival rates were calculated using the Kaplan–Meier method and the log-rank test was used to compare the variables between the groups; p values of < 0.05 were considered to indicate statistical significance.