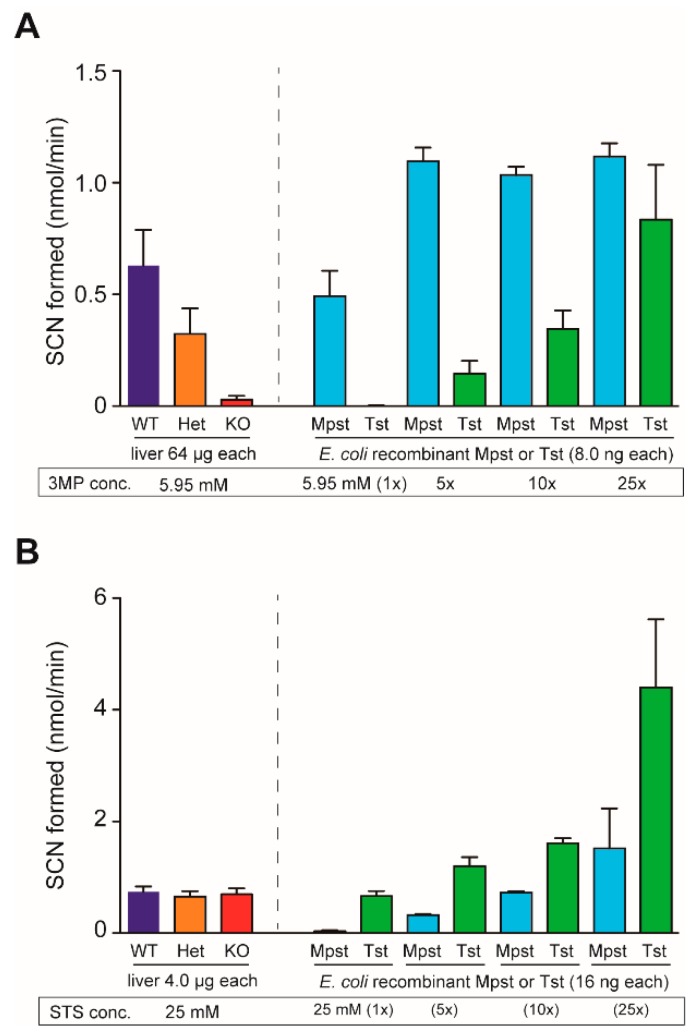
Figure 3.
Mpst and Tst (rhodanese) enzyme activities from wild-type (WT), heterozygous (Het), and homozygous (KO) Mpst mutant mice liver homogenates, as well as mouse Mpst/Tst recombinant proteins. (A) Mpst enzyme assay. Although recombinant Tst protein displayed some 3-mercaptopyruvate (3-MP) degradation “Mpst” activities at substrate concentrations over 29.75 mM (5 ×), it did not show any activity at 5.95 mM (1 ×). Under this condition, Mpst gene deletion abolished Mpst-specific activities in liver homogenates from KO mice. (B) Tst enzyme assay. Although recombinant Mpst protein displayed some sodium thiosulfate (STS) degradation “Tst” activities at >125 mM (5 ×), it did not show any activity at 25 mM (1 ×). At this condition, Mpst gene deletion did not alter Tst-specific activities at any STS concentrations tested in liver homogenates from KO mice.