Table 1.
Synthesis of dihydropyrimidone(thione)s 152–156 of antiproliferative interest mediated by Biginelli type three-component reactions.
| Reagents | Conditions | Product | Reference | ||
|---|---|---|---|---|---|
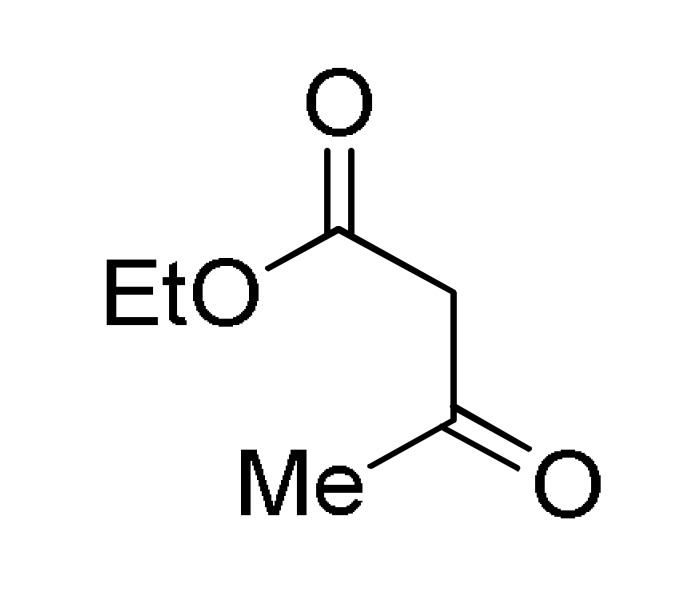 type 8 |
RCHO type 1 |
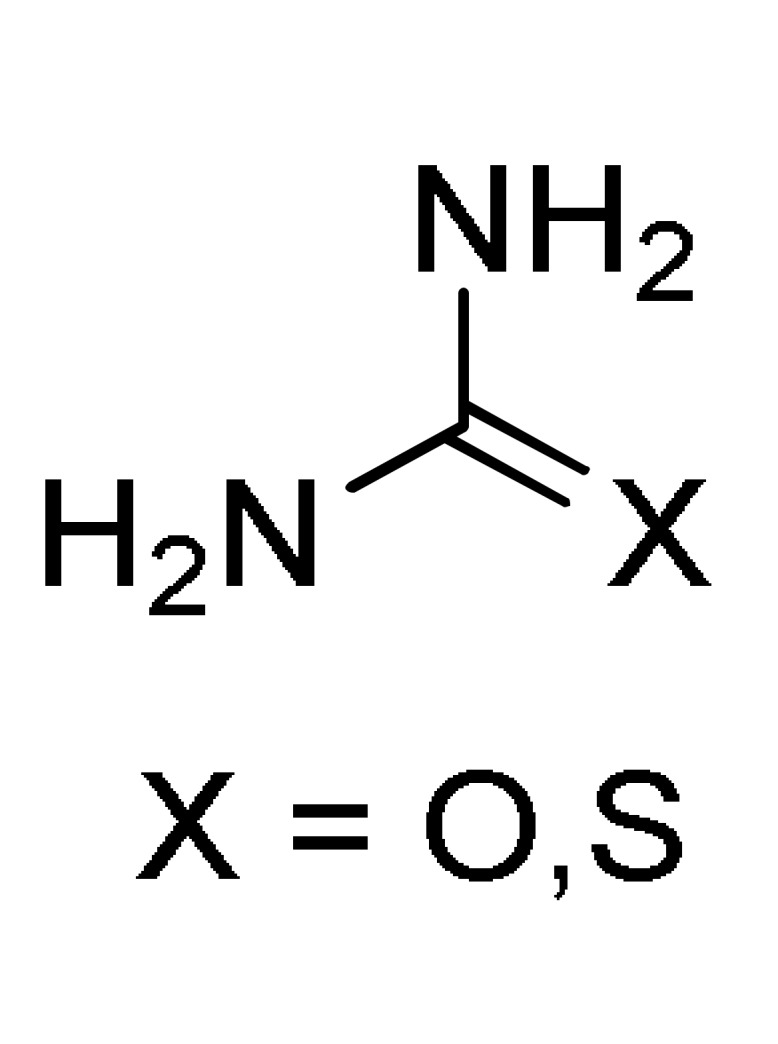
6 |
p-sulfonic acid calix[4]arene (0.5% mol), EtOH, reflux |
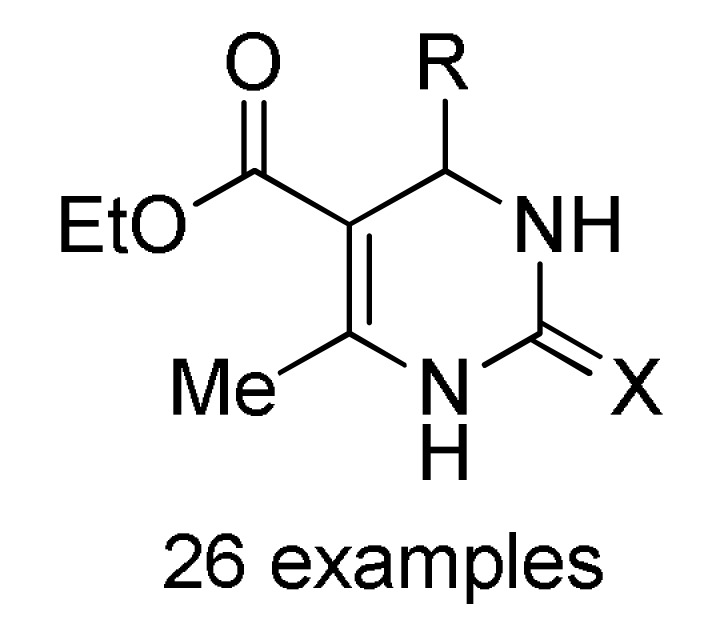
152 a |
[169] |
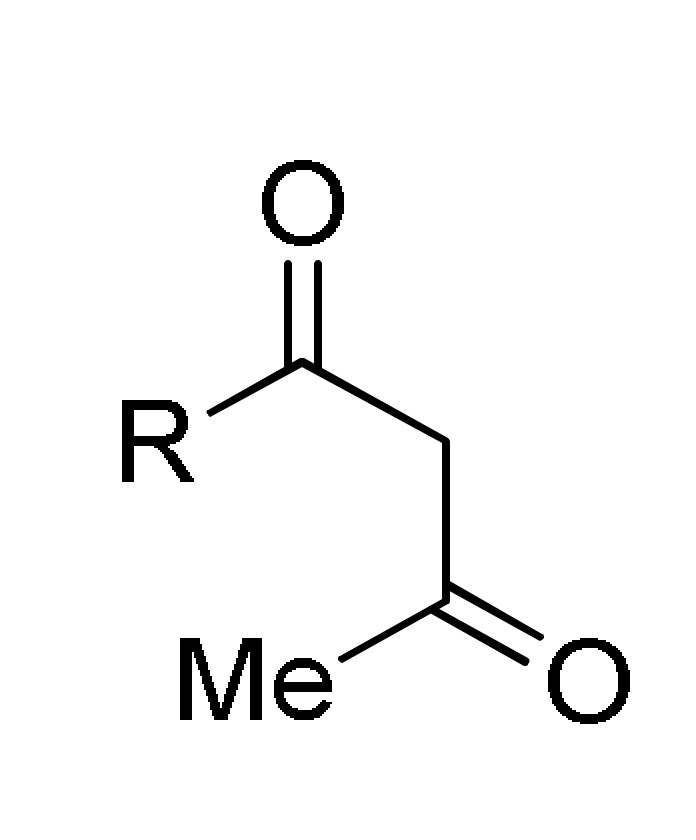 type 27 |
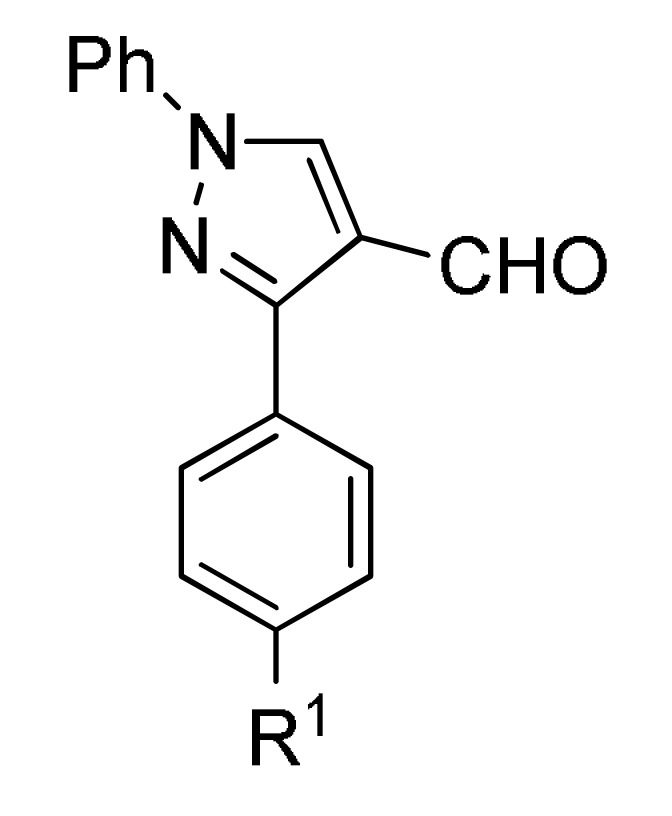 type 1 |
p-sulfonic acid, EtOH, reflux |
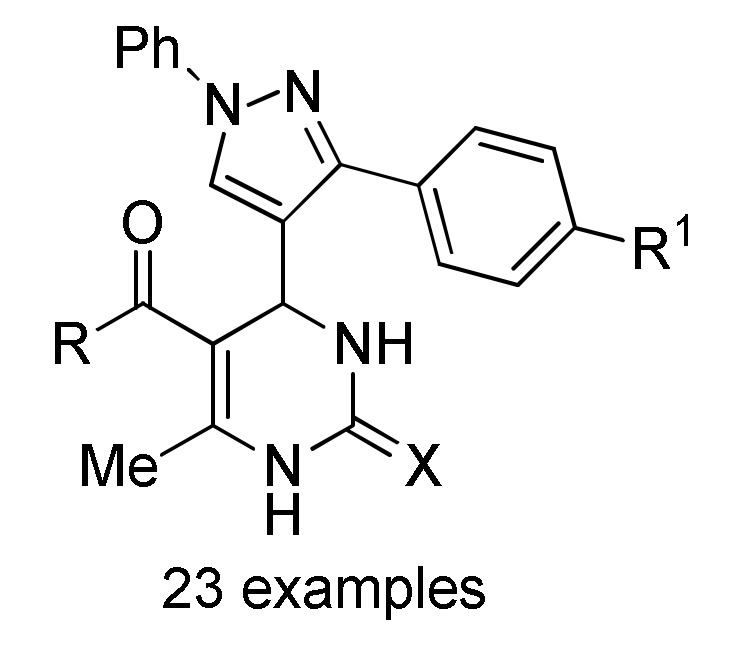
153 b |
[169] | |
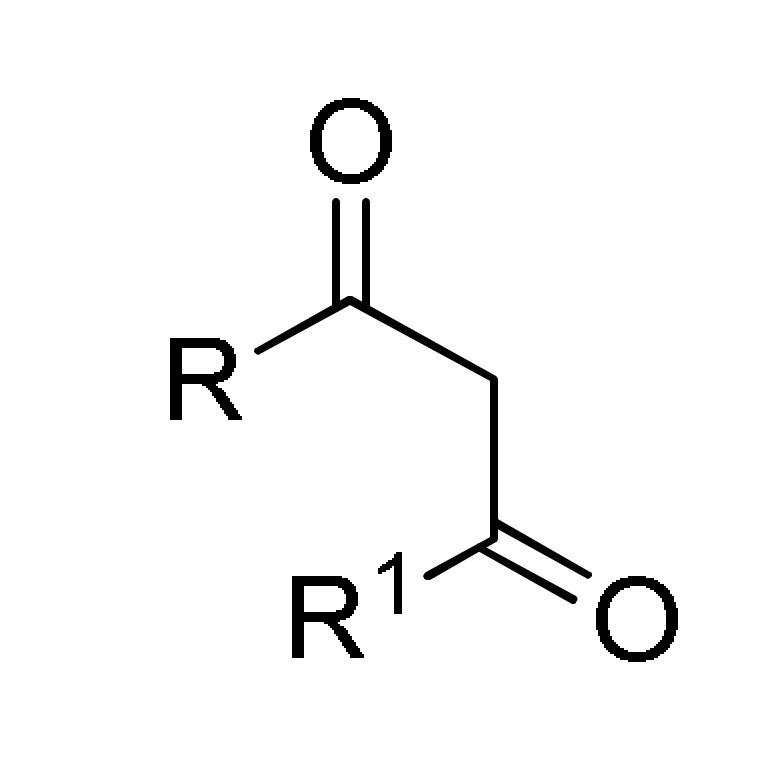 type 27 |
ArCHO/R2CHO 1 |
MAI·Fe2Cl7 (5 mol%), BMI·BF4 IL (1 mL), 80 °C |
154 c |
[170] | |
| Heterogeneous Zn- and Cd-based CPs catalysts (5 mol%), 70–100 °C, Continuous flow |
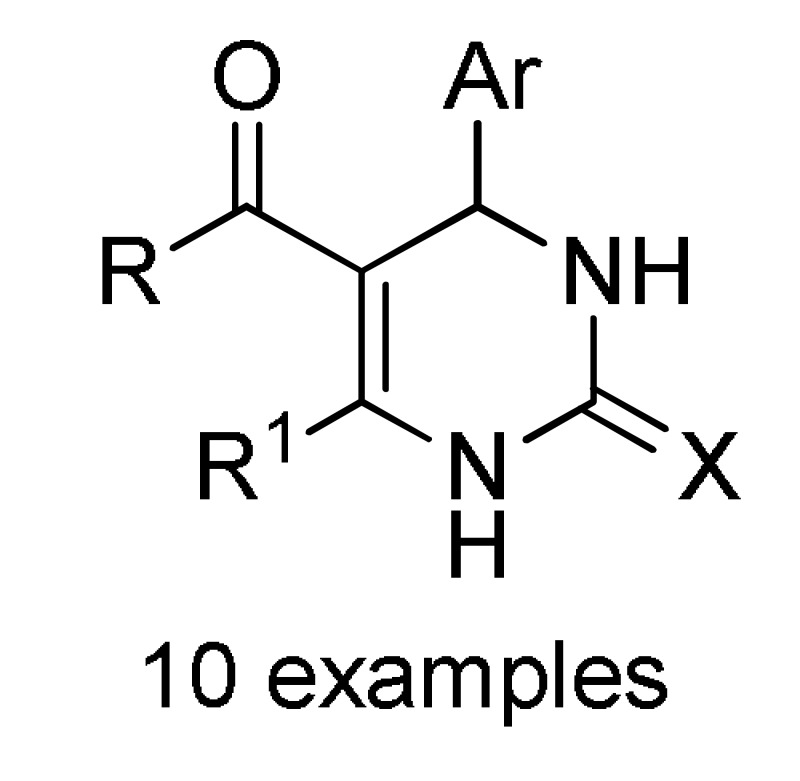
155 d |
[171] | |||
 type 27 |
 type 6 |
Bi(NO3)3.5H2O, 70 °C, solvent free |
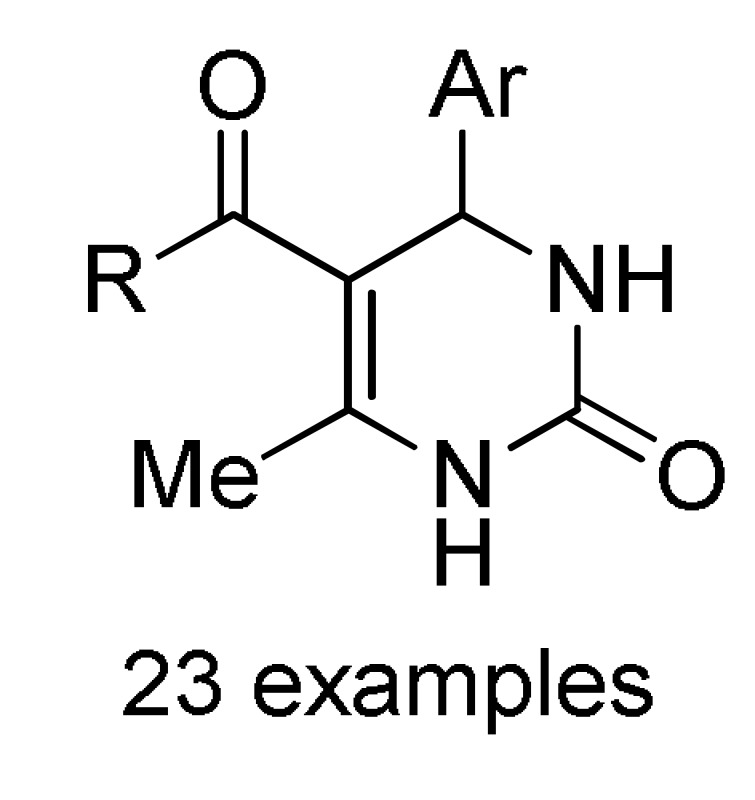
156 e |
[172] | |
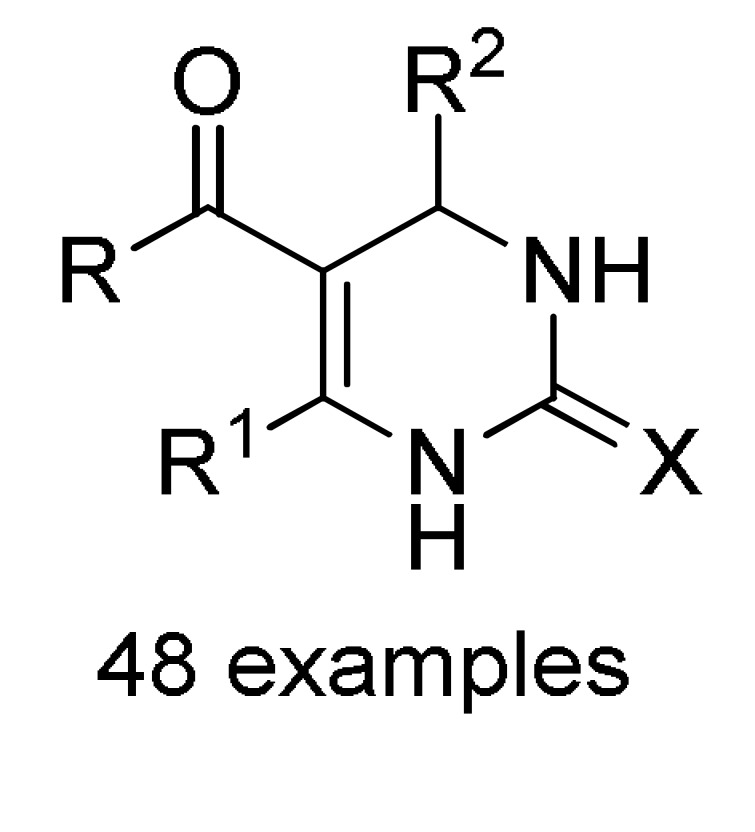
a For compounds 152: X = O, S; R = 3-HOC6H4, Ph, 4-HOC6H4, 3,4-(HO)2C6H3, 4-MeOC6H4, 3-MeOC6H4, 4-HO-3-MeOC6H3, 4-HO-3,5-(MeO)2C6H2, 4-MeSC6H4, 3,4-(OCH2O)C6H3, 4-FC6H4, Pr, cyclohexyl. b For compounds 153: X = O, S; R = OEt, PhNH, 4-MeC6H4NH, 4-MeOC6H4NH, 4-ClC6H4NH, 2-ClC6H4NH, 4-NO2C6H4NH; R1 = H, Cl. c For compounds 154: X = O, S; R = OEt, Me; R1 = Me; R2 = Ph, 4-ClC6H4, 3-HOC6H4, 2-HOC6H4, 3-NO2C6H4, 2-NO2C6H4, 4-HO-3-MeOC6H3, H, Me, 2-furyl, 3,4-(OCH2O)C6H3. d For compounds 155: X = O, S; R = OEt; R1 = Me; Ar = Ph, 3-HOC6H4, 3-NO2C6H4, 4-HO-3-MeOC6H3, 3,4-(OCH2O)C6H3. e For compounds 156: R = OMe, OEt, Me; Ar = Ph, 4-MeOC6H4, 4-MeC6H4, 4-NO2C6H4, 2,4-(Cl)2C6H3, 2,3-(Cl)2C6H3, 2,3-(F)2C6H3, 2-furyl.
