Table 2.
Multicomponent synthesis of dihydropyridine-based heterocyclic systems 157–161 of potential anticancer activity.
| Reagents | Conditions | Product | Reference | |||
|---|---|---|---|---|---|---|
| Ar1CHO 1 |
ArCOMe 16 |
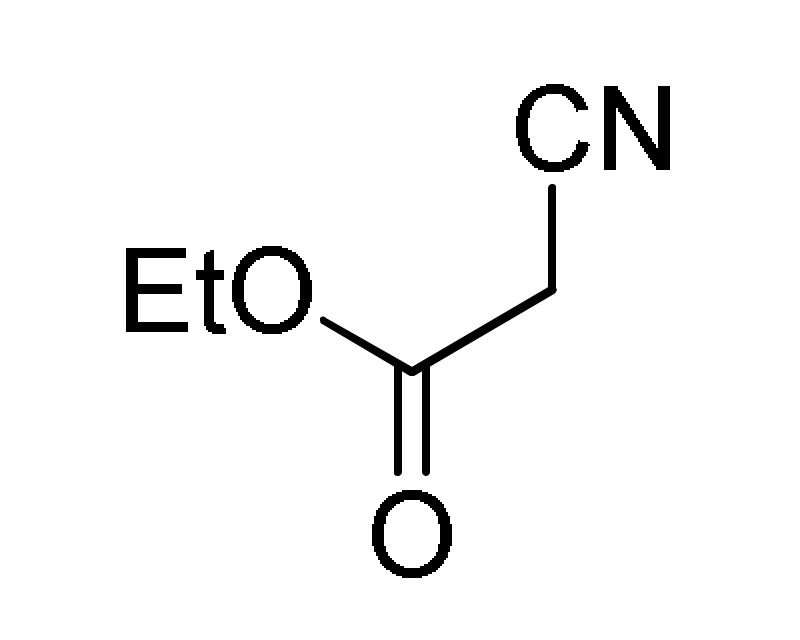
38 |
AcONH4 | EtOH, reflux |
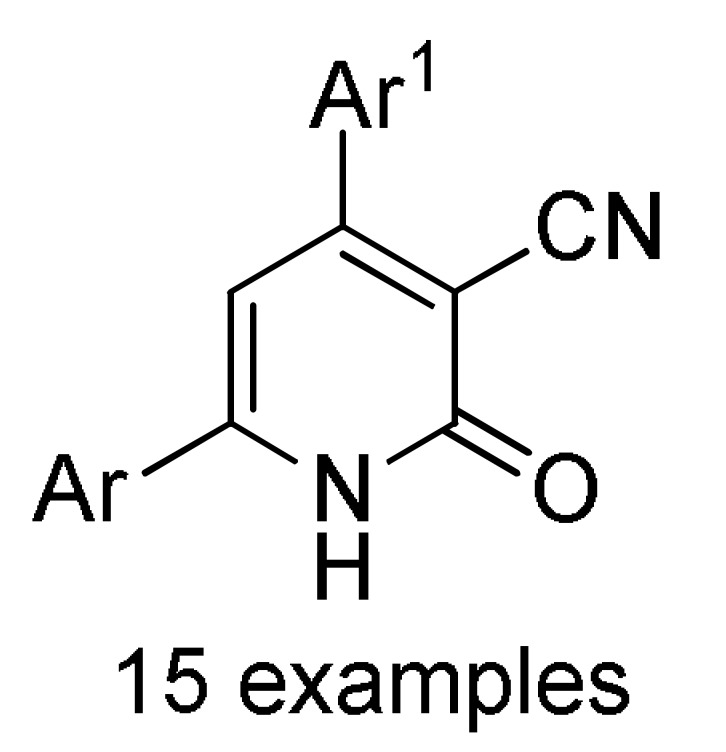
157 a |
[176] |
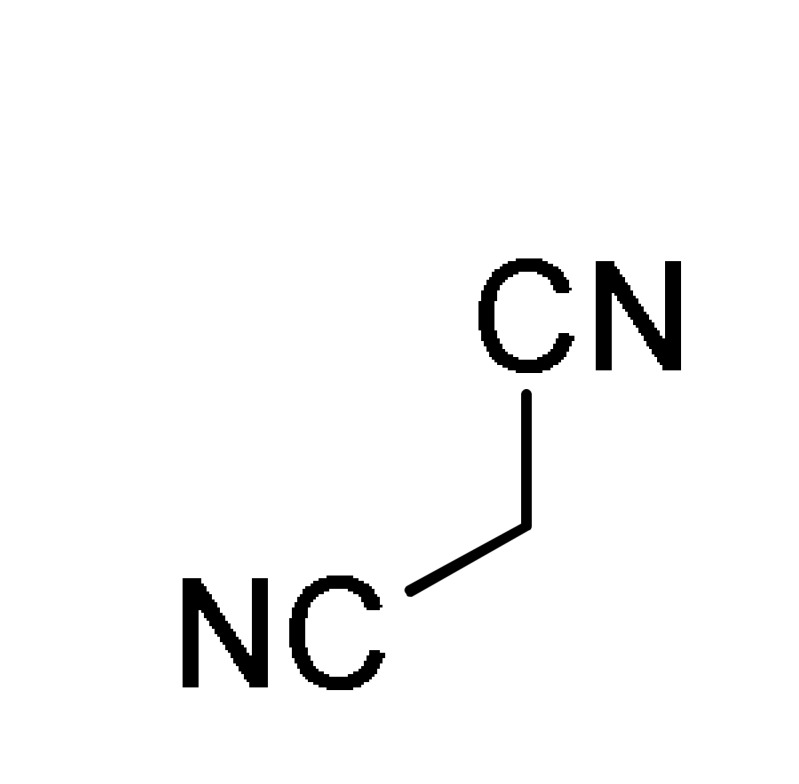
12 |
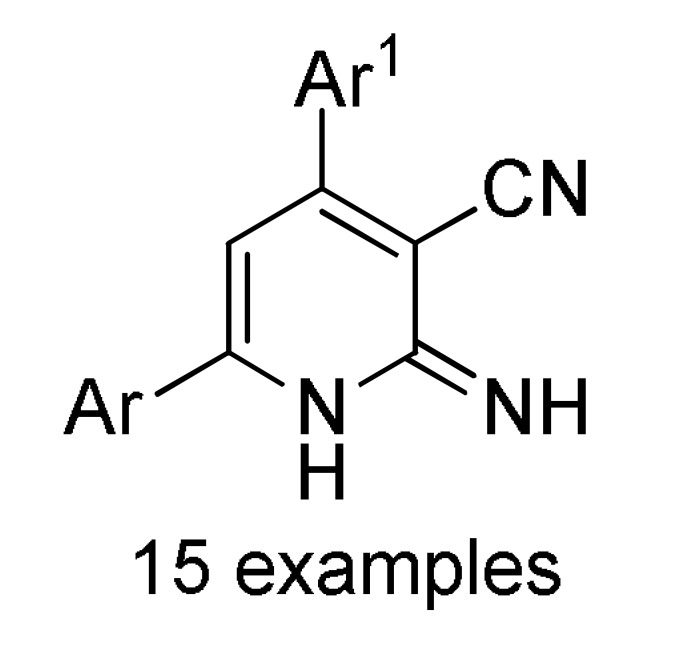
158 a |
|||||
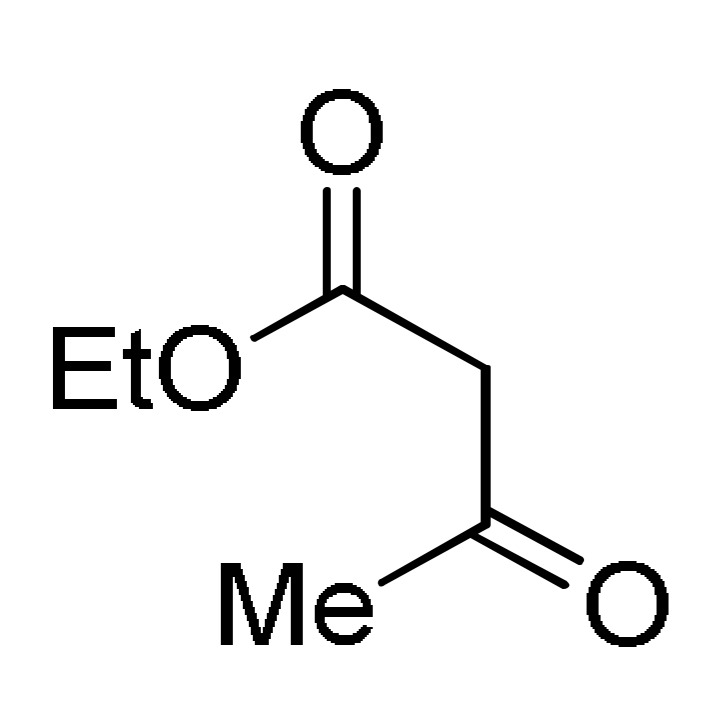
8 |
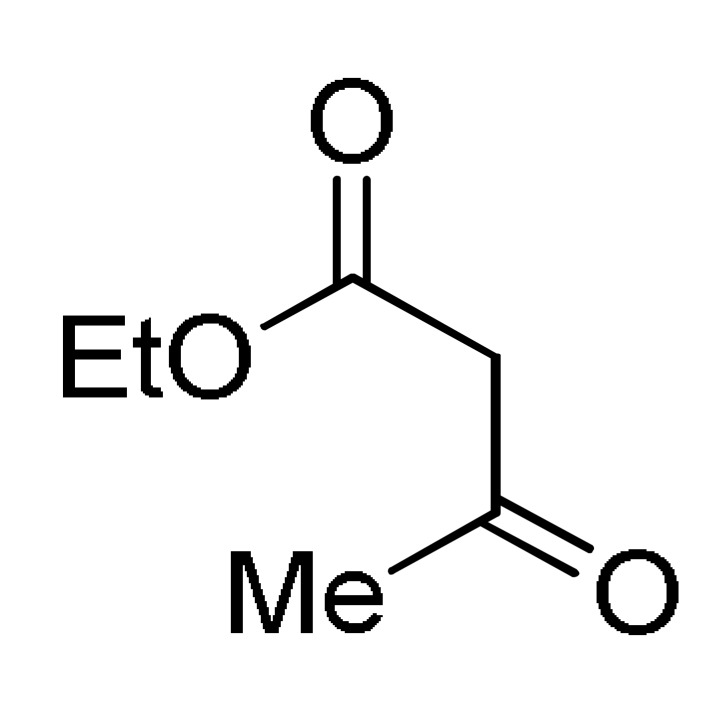
8 |
ArNH2 2 |
PEG-400, 200 W, 80 °C |
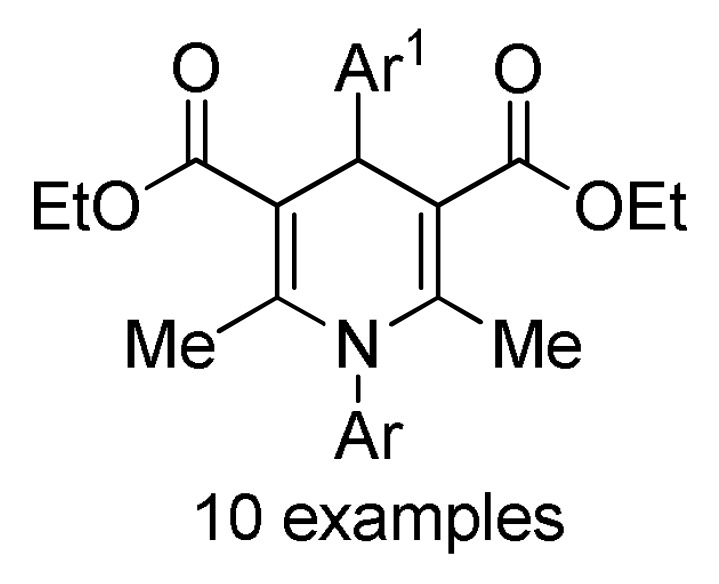
159 b |
[177] | |
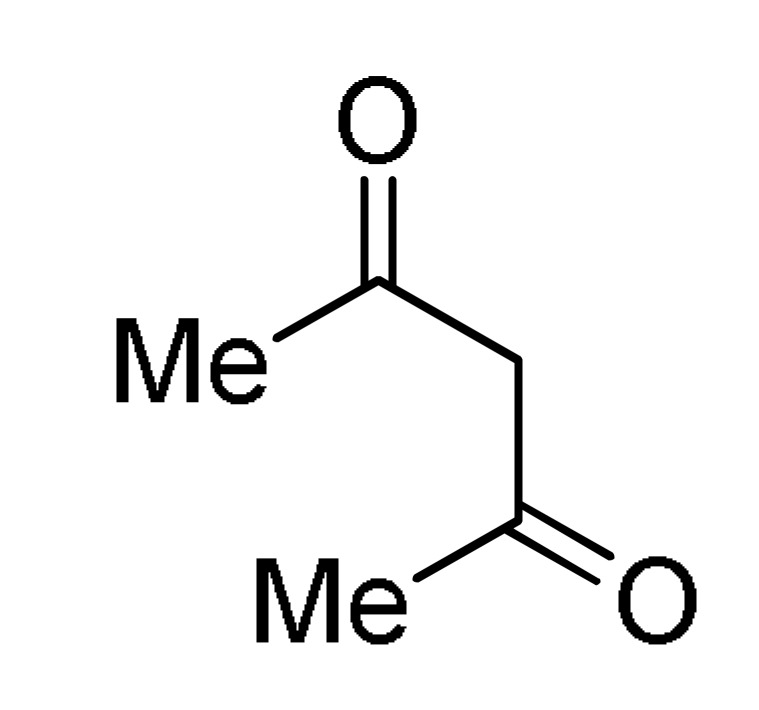
27 |
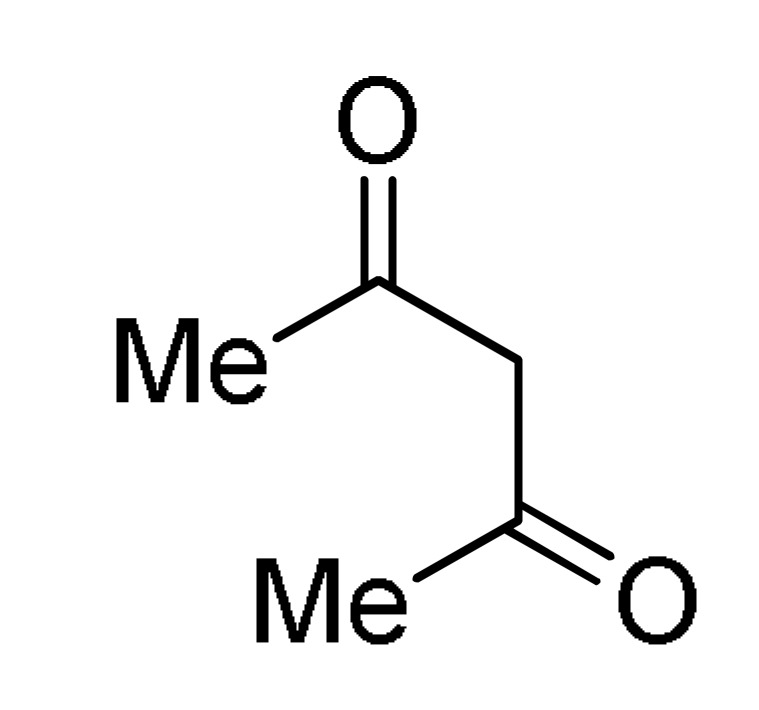
27 |
(NH4)2CO3 |
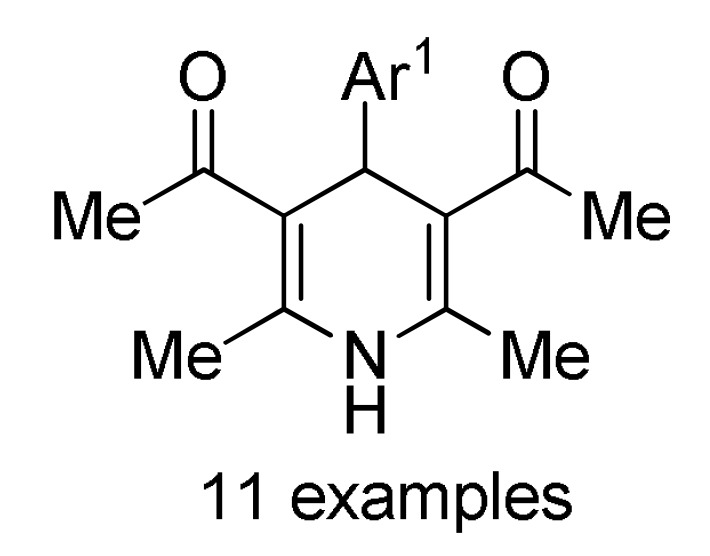
160 b |
|||
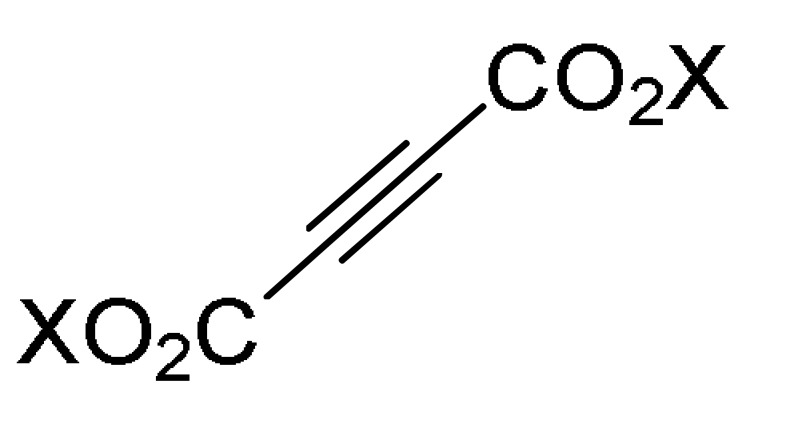
47 |
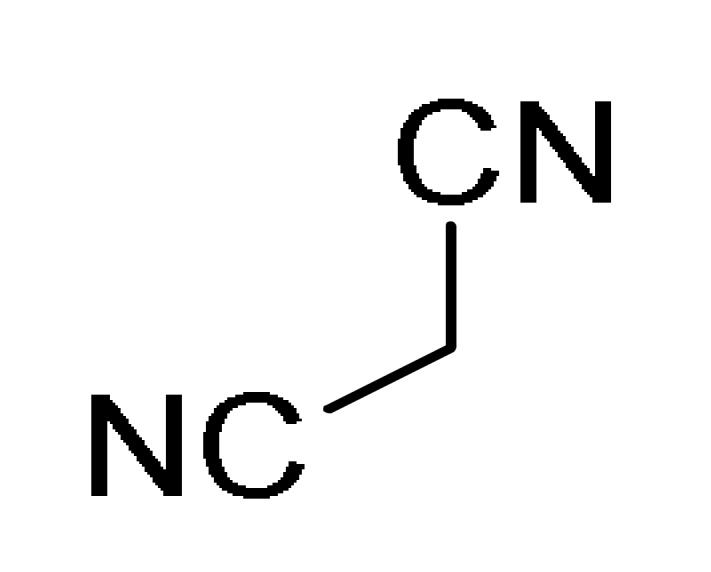
12 |
ArNH2 2 |
Montmorillonite-K10, H2O/EtOH (2:1), 60 °C |
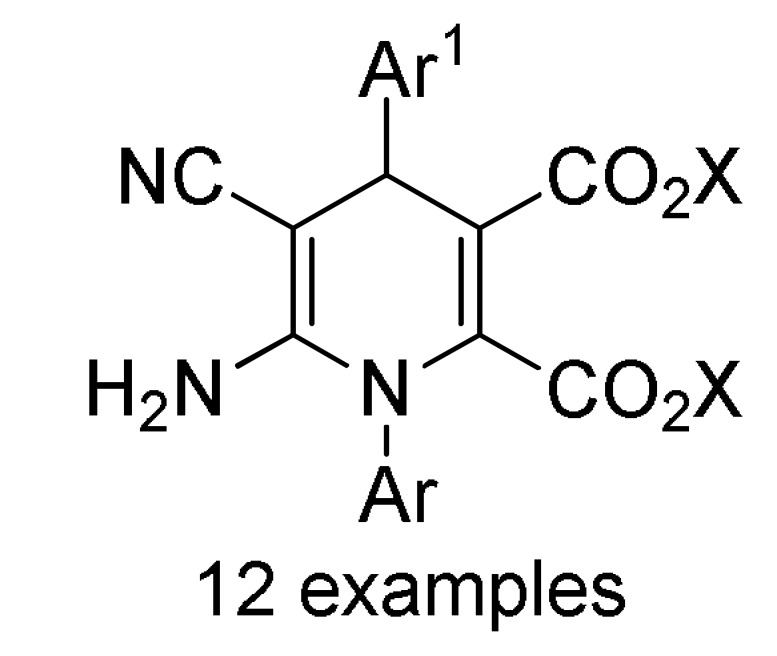
161 c |
[178] | |
a For compounds 157/158: Ar1 = 2-HOC6H4, 2-ClC6H4, 3-ClC6H4, 2,5-(MeO)2C6H3, 2,4-(MeO)2C6H3, 3-thienyl, 4-EtOC6H4, 2-EtOC6H4, 4-MeOC6H4, 2-MeOC6H4, 4-ClC6H4; Ar = 4-BrC6H4, 3-BrC6H4, 2-BrC6H4, Ph, 3-thienyl. b For compounds 159/160: Ar1 = 3-NO2C6H4, 3-PhOC6H4, 3-ClC6H4, 4-HO-3-MeOC6H3, 4-Me2NC6H4, 4-MeOC6H4, 4-ClC6H4, 4-MeSC6H4, 3-BrC6H4, 2-ClC6H4, Ph, 2-NO2C6H4, 3-MeC6H4, 2-MeC6H4; Ar = 4-MeSC6H4, 4-MeOC6H4, 3-NO2C6H4, 4-ClC6H4, 3-MeOC6H4, 4-HO-3-MeOC6H3. c For compounds 161: X = Me, Et; Ar1 = Ph, 4-BrC6H4, 3-BrC6H4, 4-MeOC6H4, 4-ClC6H4, 3-MeC6H4, 2-HOC6H4; Ar = Ph, 4-MeOC6H4, 4-BrC6H4, 4-IC6H4, 4-FC6H4, 4-NO2C6H4.
