Table 3.
Multicomponent synthesis of fused dihydroquinolines 162–165 for evaluation of their potential anticancer properties.
| Reagents | Conditions | Product | Reference | ||
|---|---|---|---|---|---|
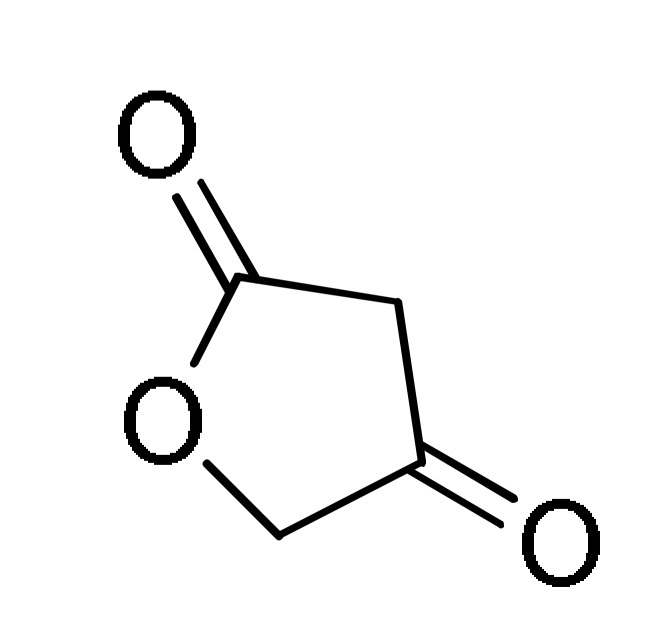
27 |
ArCHO 1 |
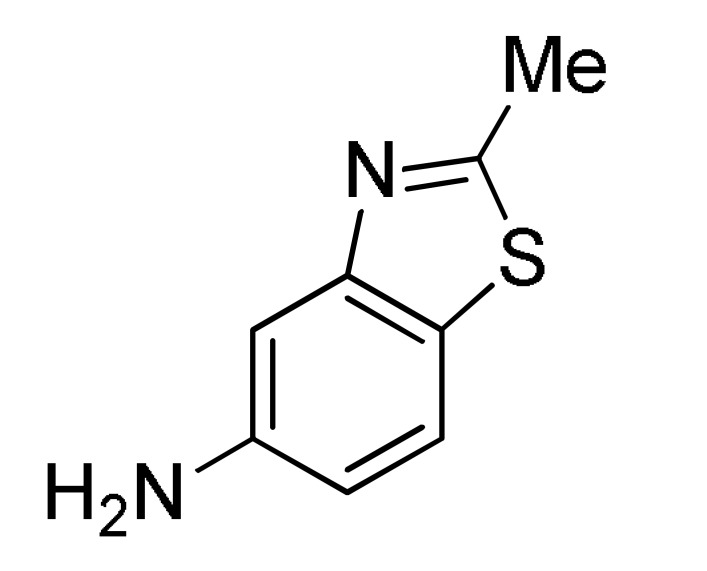
2 |
AcOH, MWI, 120 °C |
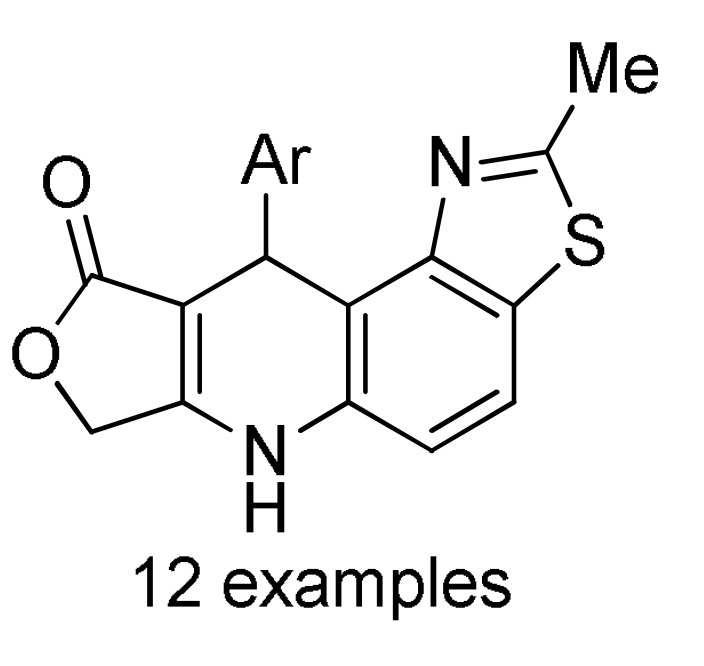
162 a |
[145] |
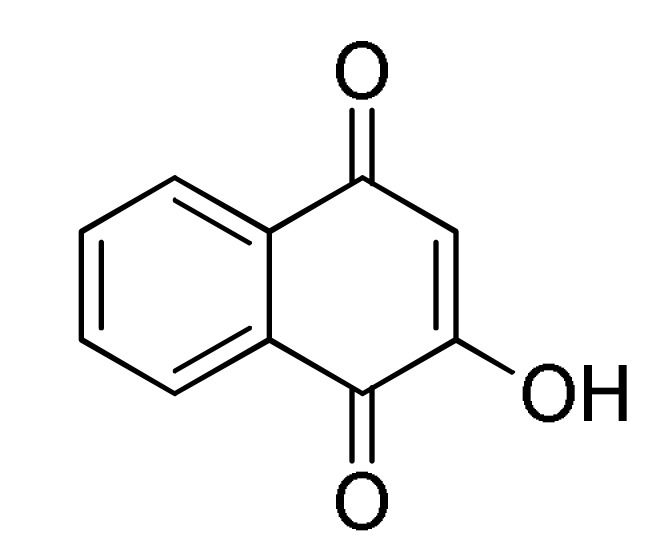
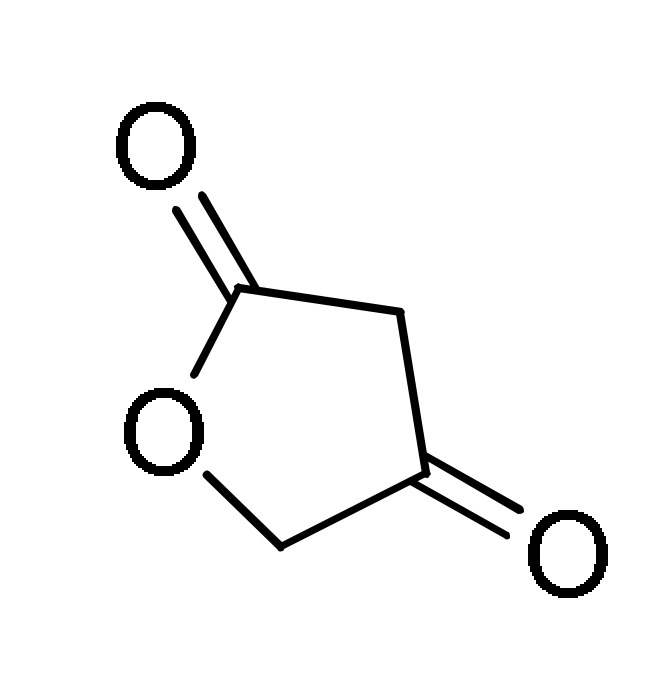
94 |
RCHO 1 |
2 |
L-Proline, EtOH, reflux |
163 b |
[181] |
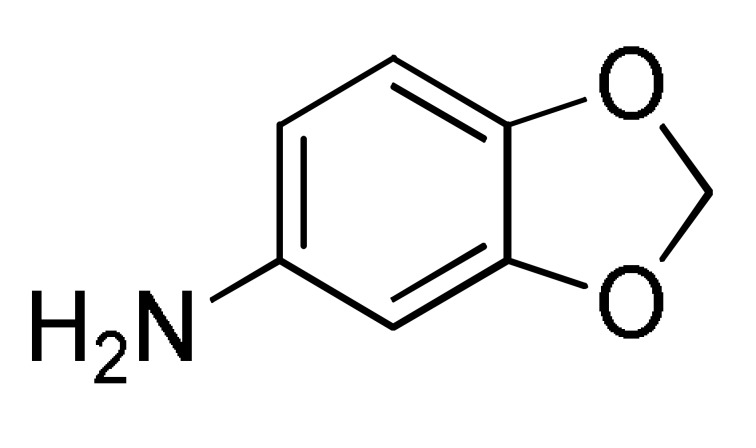
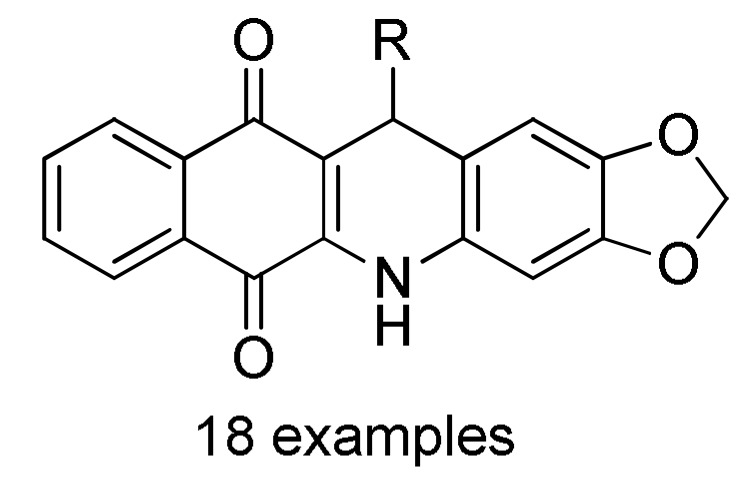
27 |
ArCHO 1 |
2 |
EtOH, reflux |
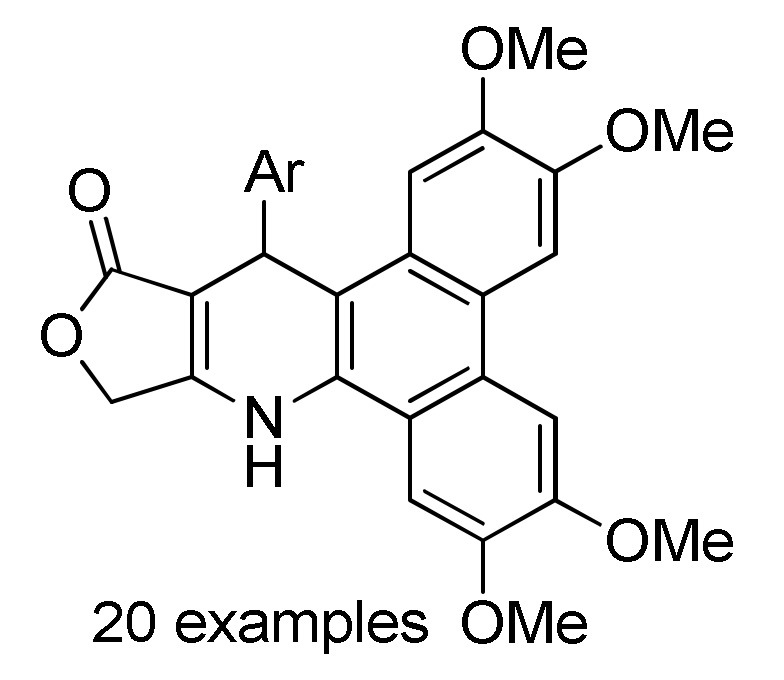
164 c |
[182] |
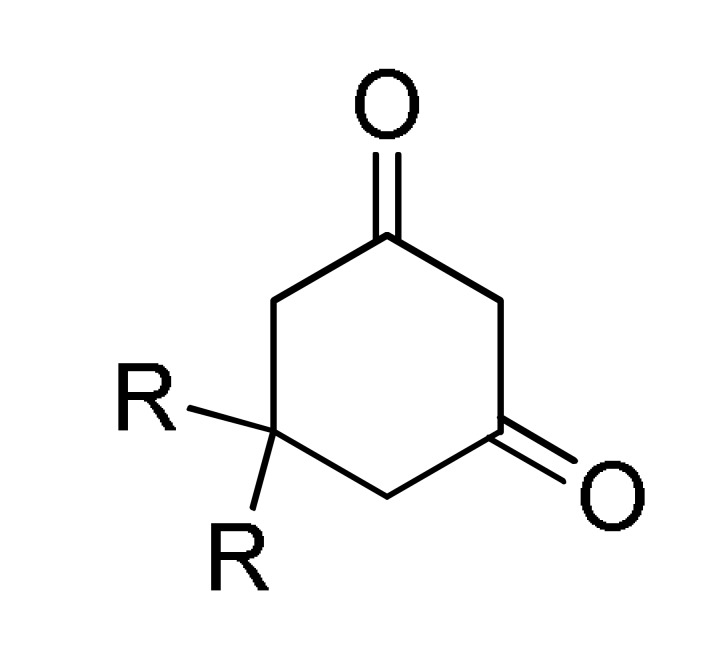
10a |
EtOH, MWI, 150 °C |
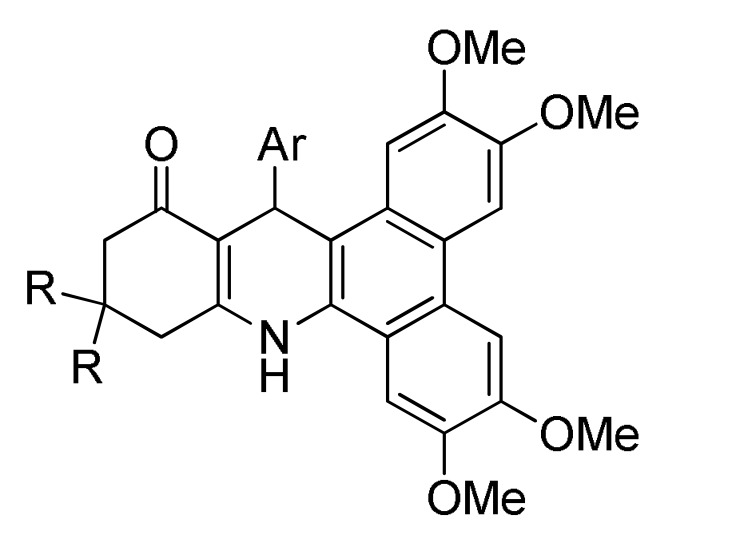
165 d |
[183] | ||
a For compounds 162: Ar = 4-FC6H4, 4-ClC6H4, 4-BrC6H4, 3-NO2C6H4, 4-NO2C6H4, Ph, 4-MeC6H4, 4-MeOC6H4, 2,4-(Cl)2C6H3, 4-HO-3-NO2C6H3, 3,4,5-(MeO)3C6H2, 2-thienyl. b For compounds 163: R = Ph, 4-MeC6H4, 4-MeOC6H4, 3-MeOC6H4, 4-ClC6H4, 2-ClC6H4, 4-FC6H4, 2-FC6H4, 3-NO2C6H4, 4-NO2C6H4, 3,4-(Cl)2C6H3, 2,4-(Cl)2C6H3, 2,5-(MeO)2C6H3, 3,5-(MeO)2C6H3, 3,4,5-(MeO)3C6H2, 2-furyl, 2-thienyl, Me. c For compounds 164: Ar = 3,4-(MeO)2C6H3, 3,4,5-(MeO)3C6H2, 2,4-(F)2C6H3, 3,4-(HO)2C6H3, 4-ClC6H4, 4-HO-3,5-(MeO)2C6H2, 4-CNC6H4, 3-BrC6H4, 4-FC6H4, 4-MeOC6H4, 3-CF3C6H4, 4-iPrC6H4, 4-MeC6H4, Ph, 2-naphthyl, 3-MeOC6H4, 2-thienyl, 3-HOC6H4, 2-FC6H4, 4-BrC6H4. d For compounds 165: R = H, Me; Ar = 4-ClC6H4, 3,4-(MeO)2C6H3, 4-CF3C6H4, 4-iPrC6H4, 3,4,5-(MeO)3C6H2, 4-BrC6H4, 4-HO-3-MeOC6H3, 4-HO-3,5-(MeO)2C6H2, 3,4-(OCH2O)C6H3, 4-MeC6H4, 4-MeOC6H4, 3-CF3C6H4, 1-naphthyl, 2-thienyl, 5-Me-2-thienyl.
