Table 4.
Multicomponent approaches for the synthesis of 7-deazaadenine (166) 7-deazapurine (167) and 7-deazahypoxanthine skeletons 168 and 169 of potential antitumor activity.
| Reagents | Conditions | Product | Reference | |||
|---|---|---|---|---|---|---|
| ArCHO 1 |
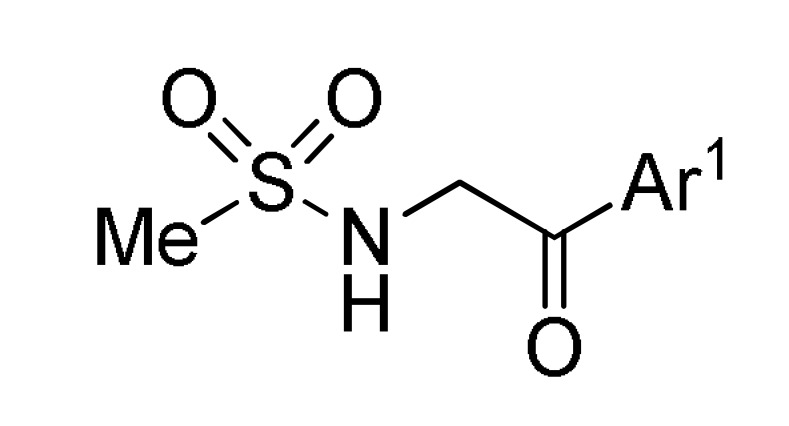
119 |
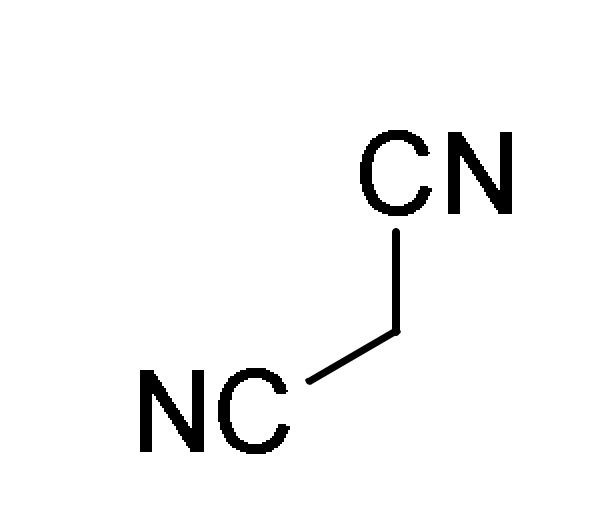
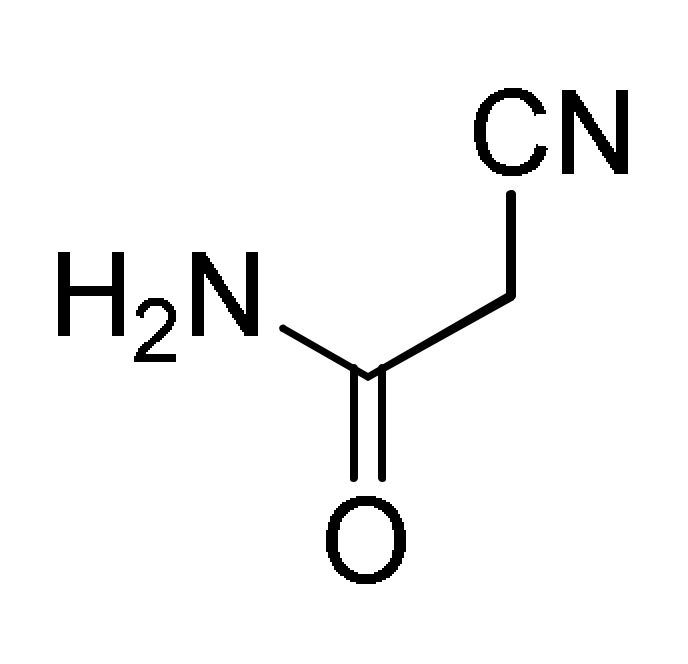
12 |
HCONH2 | K2CO3, 90 °C → 150 °C |
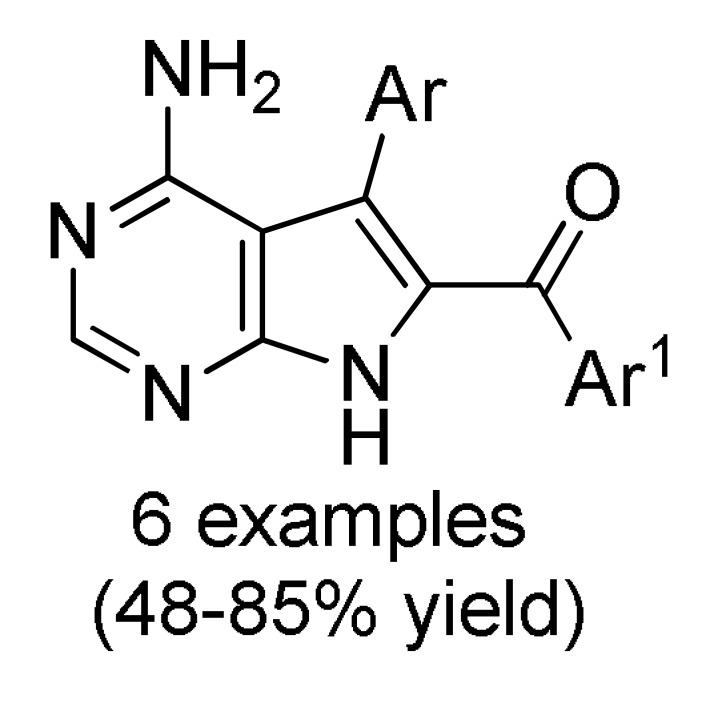
166 a |
[187] |
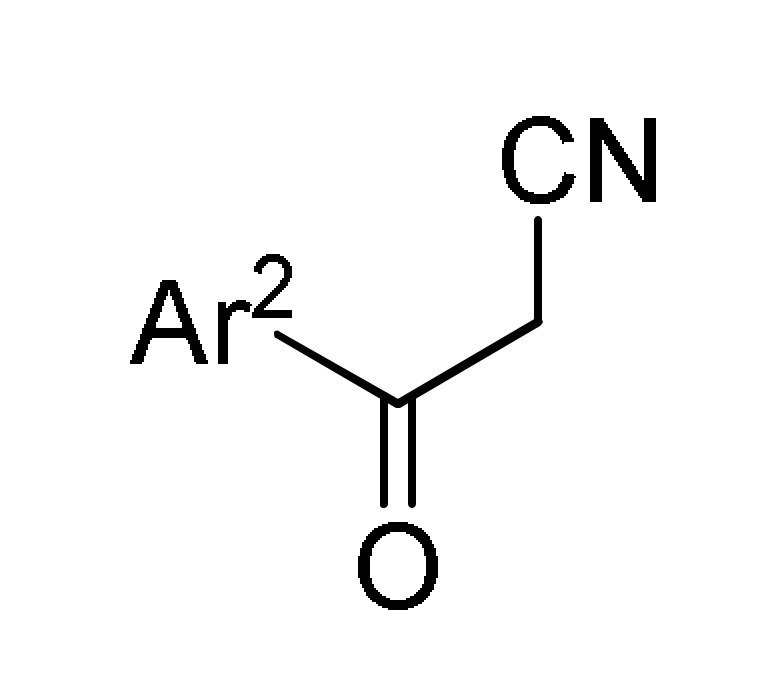
38 |
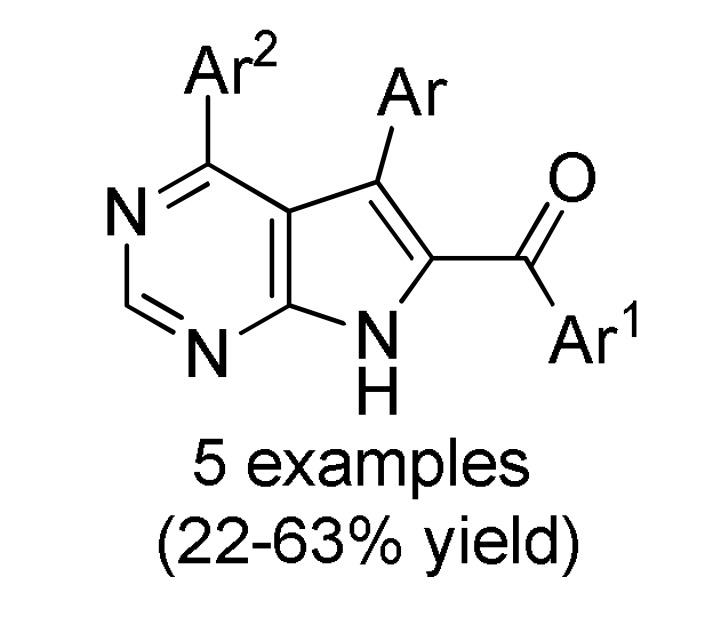
167 b |
|||||
38 |
HC(OEt)3 | EtOH, K2CO3, 90 °C → 150 °C |
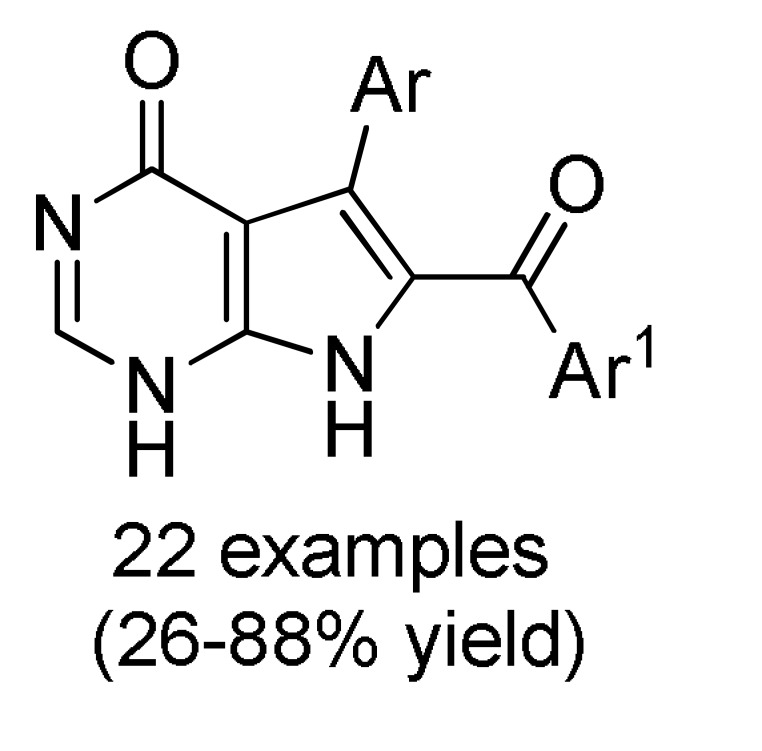
168 c |
|||
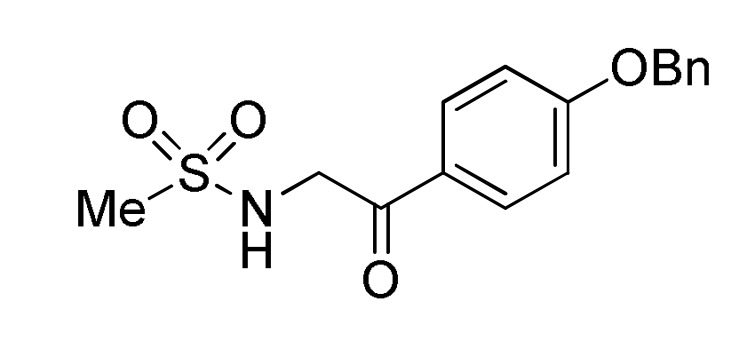
119 |
HCO2H | EtOH, K2CO3, 90 °C → 100 °C |
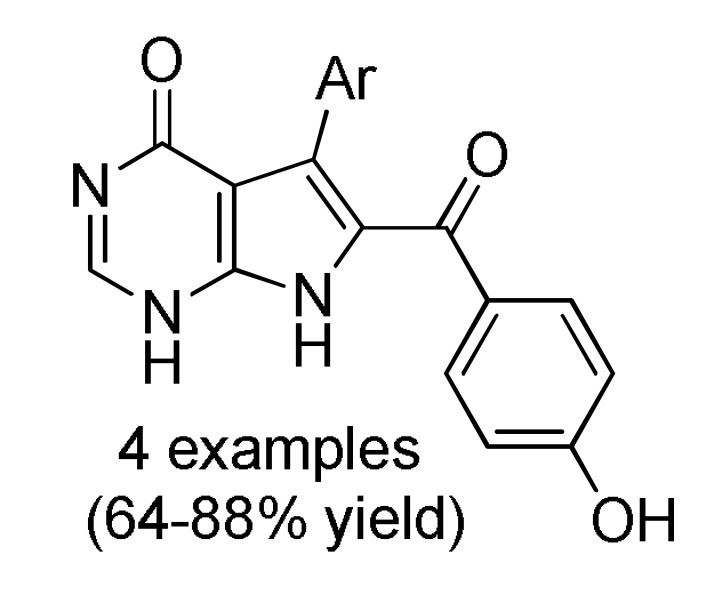
169 d |
|||
a For compounds 166: Ar = 3,5-(Br)2C6H3, 5-bromo-3-Py, Ph, 2-thienyl, 3-CNC6H4, 2,6-(Cl)2C6H3; Ar1 = Ph, 4-BnOC6H4. b For compounds 167: Ar = 4-MeOC6H4, 3,5-(Br)2C6H3, 3-Py; Ar1 = Ph, 4-MeOC6H4; Ar2 = Ph, 4-MeOC6H4, 4-MeC6H4. c For compounds 168: Ar = Ph, 3-IC6H4, 3-BrC6H4, 3-ClC6H4, 2,6-(Cl)2C6H3, 3,5-(Br)2C6H3, 3-HOC6H4, 3-CNC6H4, 3-FC6H4, 6-Br-2-Py, 5-Br-3-Py, 3-Py, 3,4,5-(MeO)3C6H2, 3,4-(MeO)2-5-IC6H2, 5-Br-3,4-(MeO)2C6H2, 4-Br-2-thienyl; Ar1 = Ph, 4-MeOC6H4, 4-FC6H4, 4-BnOC6H4, 4-BrC6H4. d For compounds 169: Ar = Ph, 3,5-(Br)2C6H3, 4-HOC6H4, 4-HO-3-MeOC6H3.
