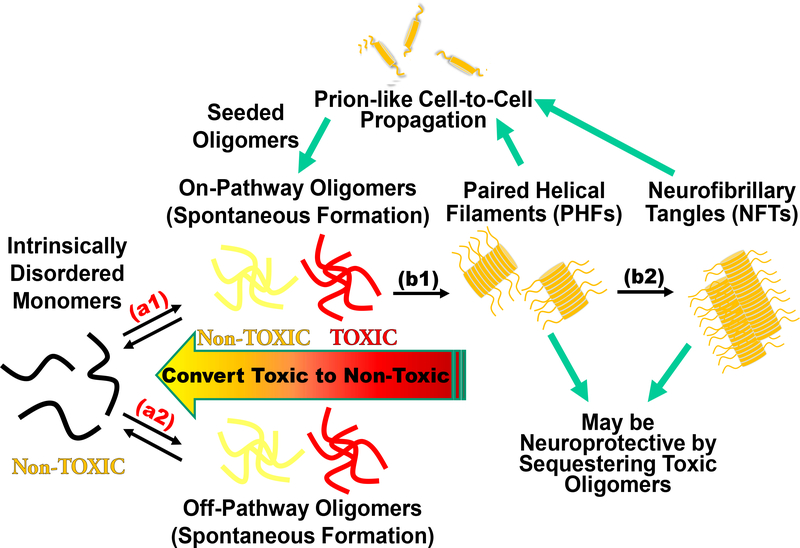
Fig. 1. Tau fibrillogenesis cascade for tauopathies and Alzheimer’s disease (AD).
The intrinsically disordered tau monomer is capable of misfolding into spontaneously formed oligomers, producing toxic assemblies implicated in AD (arrows a1 and a2). While oligomers are metastable and difficult to monitor with high precision and accuracy, the large assemblies (paired helical filaments (PHFs) and neurofibrillary tangles (NFTs), arrows b1 and b2) form irreversibly with β-sheet structures. The fibrillar species can be excreted via exosomes leading to a prion-like cell-to-cell propagation of pathology and may induce seeded oligomerization. NFTs may be neuroprotective by sequestering toxic oligomers and disruption of NFTs may induce toxicity from elevated concentrations of toxic oligomers. Our cellular time-resolved fluorescence resonance energy transfer (FRET) biosensors target reversible spontaneous oligomerization (a1 and a2), while also monitoring seeded aggregation and downstream processes such as fibrillization (b1 and b2) with high sensitivity.