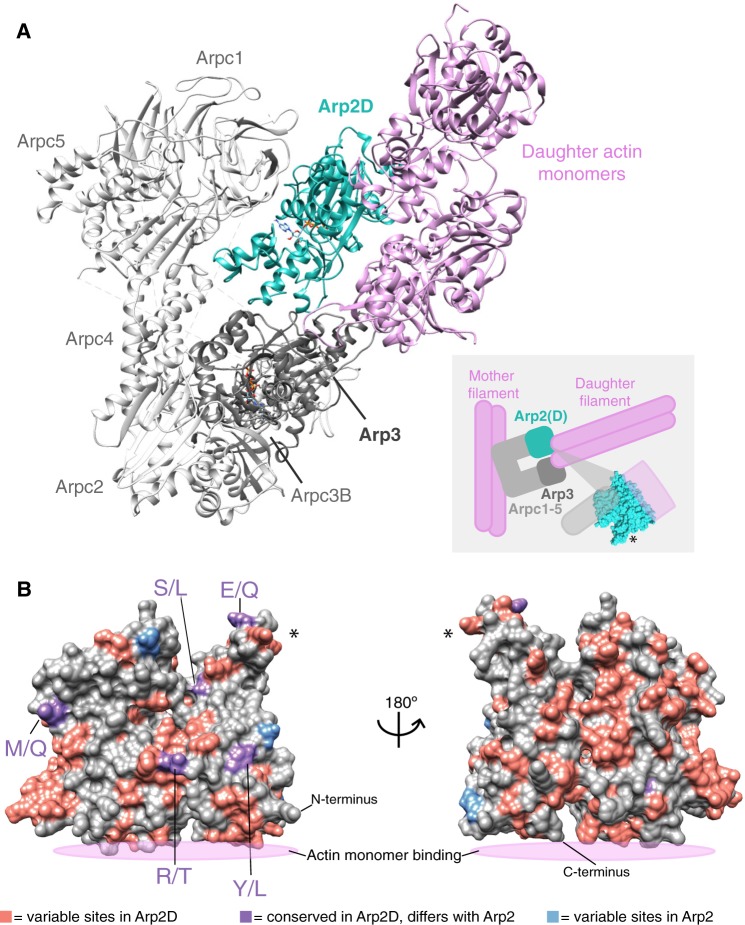
Fig. 4.
Arp2D is predicted to bind a daughter actin monomer. (A) A homology model of the Drosophila pseudoobscura Arp2/3 complex bound to daughter actin monomers was subjected to structural refinement and equilibration with Arp2D replacing Arp2. Components of the complex are labeled, with daughter actin monomers in pink and Arp2D in teal. The cartoon inset depicts how the canonical Arp2/3 complex binds to a pre-formed “mother” actin filament and subsequently generates a daughter filament, with Arp2/3 at the junction. The structure of Arp2 is shown in the inset to indicate where the interaction with actin and the complex approximately takes place. An asterisk on one part of the structure in the inset serves as a reference point for (B). (B) Drosophila pseudoobscura Arp2 is shown as a space-filled homology model. All Arp2D protein sequences and a few representative Arp2 sequences (from D. persimilis, D. pseudoobscura, D. guanche, D. bifasciata, and D. azteca) were aligned and residues were categorized as one of the following: 1) variable sites in Arp2D (pink), 2) conserved in Arp2D but differs from conserved residues in Arp2 (purple, denoted as Arp2 residue/Arp2D residue), and 3) variable sites in Arp2 residues (blue). The asterisk marks the same subdomain of the structure shown in (A).