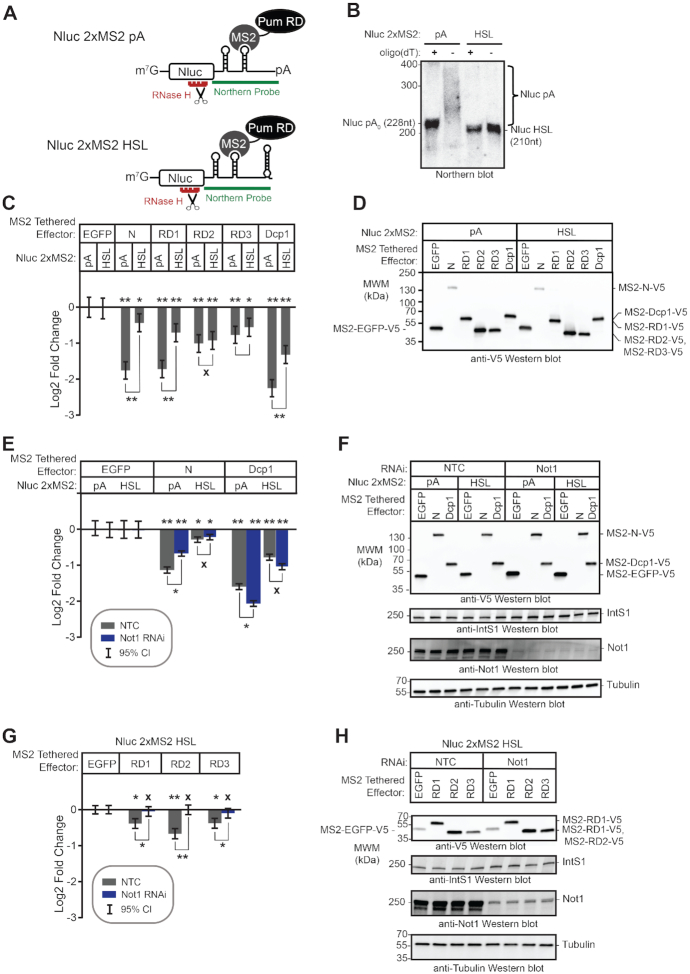
Figure 8.
The poly(A) tail is necessary for maximal activity of the Pum N-terminus. (A) Diagram of the Nluc 2×MS2 reporters with either 3′ poly(A) tail or the Histone Stem Loop (HSL) used for tethered function assays. The reporters are identical except that the cleavage/poly-adenylation element was replaced with a Histone Stem Loop and Histone Downstream Element (HDE) in the Nluc 2× MS2 HSL reporter, which produces a non-adenylated 3′ end. The location of the probe used for Northern blotting (green) and the DNA oligonucleotide (red) used for RNase H cleavage of the mRNAs for high resolution Northern blotting are indicated. Diagram is not drawn to scale. (B) High resolution Northern blot of the Nluc 2× MS2 pA and HSL reporter mRNAs expressed in d.mel2 cells confirms proper poly-adenylation of the pA reporter and lack of poly-adenylation of the HSL reporter. Where indicated (+), RNA was treated with DNA oligonucleotide of 15 thymidines (dT) and RNase H to degrade the poly(A) tail. RNA size markers are indicated on the left. The lengths of the 3′ end reporter fragments are also indicated. (C) The repression activity of the Pum N-terminus and RDs was measured via tethered function dual luciferase assay, using the Nluc 2×MS2 poly(A) and HSL reporters. The activity of each effector was determined relative to the tethered EGFP negative control effector on the same reporter. Mean log2 fold change and 95% credible intervals are reported in the graph for three experimental replicates with four technical replicates each. Data and statistics are reported in Supplementary Table S1. For significance calling, a ‘*’ denotes a posterior probability >0.95 that the difference relative to the negative control is in the indicated direction. The ‘**’ indicates a posterior probability of >0.95 that the indicated difference is at least 1.3-fold. An ‘x’ marks a posterior probability >0.95 that the indicated difference is no more than 1.3-fold in either direction. (D) Western blot detection of the V5-epitope tagged MS2 fusion effector proteins used in panel C from a representative experimental replicate. Equivalent mass of protein from each sample was probed with anti-V5 antibody. (E) The effect of RNAi-mediated depletion of Not1 on repression activity of the Pum N-terminus was measured via tethered function, using the Nluc 2× MS2 poly(A) and HSL reporters. The non-targeting control (NTC) served as negative control for RNAi. The repression activity of each effector was determined relative to tethered EGFP negative control in the same RNAi condition. The mean log2 fold change and 95% credible intervals are graphed for three experimental replicates with four technical replicates each. Data and statistics are reported in Supplementary Table S1. (F) Western blot of the V5-epitope tagged MS2 fusion effector proteins from a representative experimental replicate from panel E. Equivalent mass of protein from each sample was probed with anti-V5 antibody or anti-IntS1 to assess equal loading of lanes. The depletion of Not1 protein was assessed using an anti-Not1 antibody, with anti-tubulin western blot serving as the loading control. (G) The effect of RNAi depletion on the repression activity of the Pum RDs was measured via tethered function dual luciferase assay, using the Nluc 2× MS2 HSL reporter. Non-targeting control (NTC) serves as negative control for comparison. Activity of each effector was determined relative to tethered EGFP negative control in the same RNAi condition. The mean log2 fold change and 95% credible intervals are reported in the graph for three experimental replicates with four technical replicates each. Data and statistics are reported in Supplementary Table S1. (H) Western blot of the V5-epitope tagged MS2 fusion effector proteins used in from a representative experimental replicate from pane G. An equivalent mass of protein from each sample was probed with anti-V5 antibody, and then with anti-IntS1 to assess equal loading of lanes. Depletion of the Not1 protein was assessed using an anti-Not1 antibody, with ant-tubulin western blot serving as a loading control.