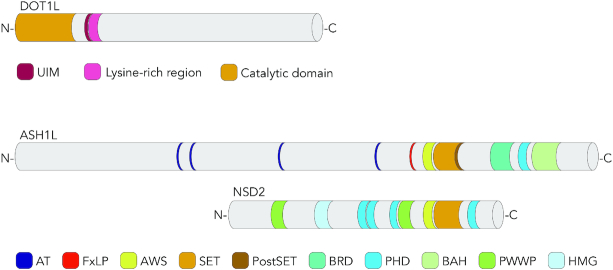
Figure 3.
Overview of histone KMTs stimulating the GGR reaction in response to UV damage. DOT1L is the only known seven-β-strand methylase able to methylate lysine residues. Its catalytic domain, containing the seven-β-strand fold, is flanked by a ubiquitin-interacting motif (UIM) mediating its binding to ubiquitinated H2B (152,225,226). The lysine-rich region also participates in the interaction with ubiquitinated H2B and contributes to H3K79 methylation (227). ASH1L and NSD2 are members of a large family of SET-domain methyltransferases (146). The SET domain flanked by AWS (Associated With SET) forms the catalytic site. The post-SET motif displays a flexible auto-inhibitory loop (188–190). Further domains are the ‘AT hooks’ (putative DNA-binding motifs), BRD (a BRomoDomain interacting with acetylated histones), PHD (Plant HomeoDomain for the binding to methylated histones), BAH (a Bromo-Associated Homology domain for the binding to methylated histones and other proteins), PWWP (Pro-Trp-Trp-Pro domain for the binding to methylated histones) and an HMG box (for High Mobility Group, shown to interact with the DNA binding domain of the androgen receptor) (146,152,228,229). The FxLP motif in ASH1L mediates interactions with MRG15, a chromodomain protein stimulating ASH1L activity by releasing its auto-inhibitory loop.