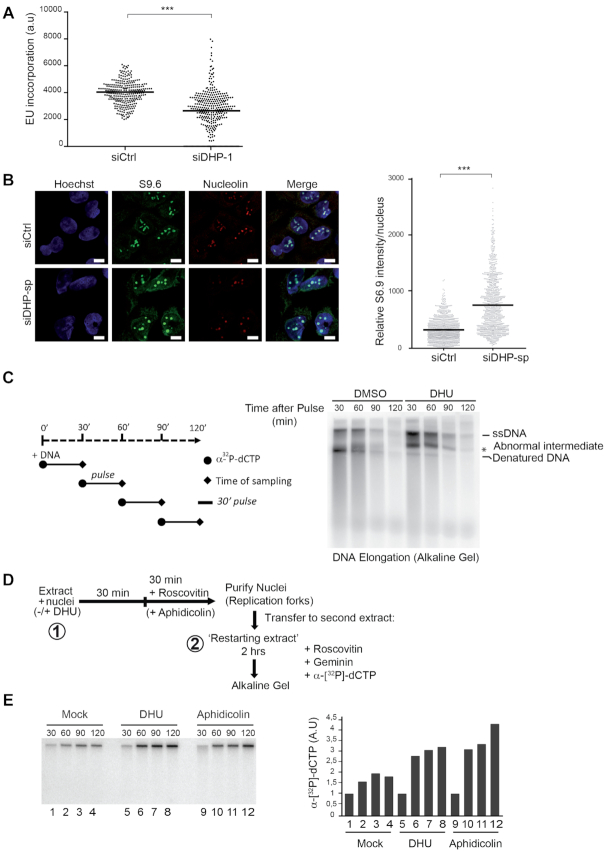
Figure 4.
Dihydropyrimidines induce transcriptional stress and yield abnormal DNA replication intermediates. (A) Graphic representation of global transcriptional activity visualized by 5-ethynyl uridine (EU) incorporation. U-2 OS cells transfected with control and anti-DHP siRNA (siDHP-1) were labeled with EU for 20 min before fixation. The EU intensity of 100 cells from two independent biological replicates was measured by fluorescence microscopy. The bar dissecting the data points represents the median of EU intensity. Differences between distributions were assessed with the Mann–Whitney rank sum test. P-values: *** < 0.0001. (B) Immunofluorescence staining with S9.6 and nucleolin antibodies of DHP-depleted or control U-2 OS cells (siDHP-sp). DNA was stained by Hoechst. Bars indicate 10 μm. Right panel: The graph shows the median of S9.6 signal intensity par nucleus after nucleolar signal removal. More than 1000 cells from two independent biological replicates were considered. Differences between distributions were assessed with the Mann–Whitney rank sum test. P-values: *** < 0.0001. (C) Left panel: Experimental scheme. DNA synthesis reactions (control: DMSO; DHU: 15 mM) were pulse-labeled for 30 min with α-[32P]-dCTP at the indicated times during the course of a 2-h reaction. Replication products were purified and resolved by electrophoresis through a 1.2% agarose gel in denaturing conditions. (*) abnormal replication intermediate. One representative experiment is shown from two biological replicates. (D) Experimental scheme: Sperm nuclei were added to Xenopus egg-extract (in presence or not of 7.5 mM DHU dissolved in water) and incubated at 23°C to allow origins firing and replication initiation. After 30 min incubation, the firing of new replication origins was blocked with roscovitine (0.5 mM). Replicating nuclei were then isolated after 60 min of incubation and transferred to a second extract (restarting extract) supplemented with roscovitine (0.5 mM) and Geminin (60 mM) to block the firing and the assembly of novel origins, respectively. DNA synthesis reactions were pulse-labeled with α-[32P]-dCTP during incubation in the second extract. (E) Replication products were resolved by 1% alkaline agarose gel electrophoresis and revealed by autoradiography. Lanes 1–4: Mock treated extracts; Lanes 5–8: incubation in the first extract was performed in the presence of 7.5 mM DHU. Lanes 10–12 serve as positive controls: after 30 min incubation in the first extract, DNA synthesis was blocked with aphidicolin (100 ng/μl). Right panel: Histogram representing the quantification of the gel by image J of replication products (arbitrary unit). One representative experiment is shown from two biological replicates (See Supplementary Figure S4A).