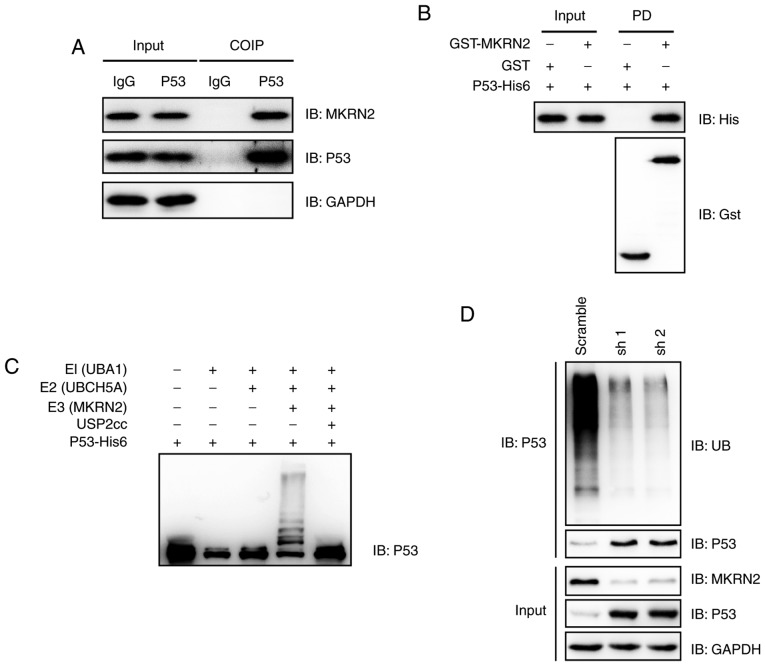
Figure 4.
MKRN2 interacts with and ubiquitylates P53. (A) Endogenous MKRN2 and P53 formed a complex in A375 cells. A375 cell lysates were immunoprecipitated with anti-P53 antibody and subjected to immunoblotting analysis. (B) MKRN2 interacted with P53 in vitro. Recombinant GST-tagged MKRN2 and His6-tagged P53 were purified, and then GST pull-down assays were performed and subjected to immunoblotting analysis. (C) MKRN2 ubiquitylated P53 in vitro. An in vitro ubiquitylation assay was carried out with the recombinant proteins P53-His6, E1 (UBA1-His6), E2 (UBCH5A-His6), His6-Ub and Gst-MKRN2, together with the indicated components, and subjected to immunoblotting analysis. (D) MKRN2 ubiquitylated P53 in vivo. A375 cells stably transfected with MKRN2 shRNAs were lysed in RIPA buffer and immunoprecipitated with anti-P53 antibody and subjected to immunoblotting analysis using the indicated antibodies. COIP, co-immunoprecipitation; GST, glutathione S-transferase; His, histidine; IgG, immunoglobulin G; MKRN2, makorin ring finger protein 2; sh, short hairpin RNA; shRNA, short hairpin RNA; UBA1, ubiquitin like modifier activating enzyme 1; UBCH5A, ubiquitin conjugating enzyme E2 D1; USP2, ubiquitin specific peptidase 2; IB, immunoblot.