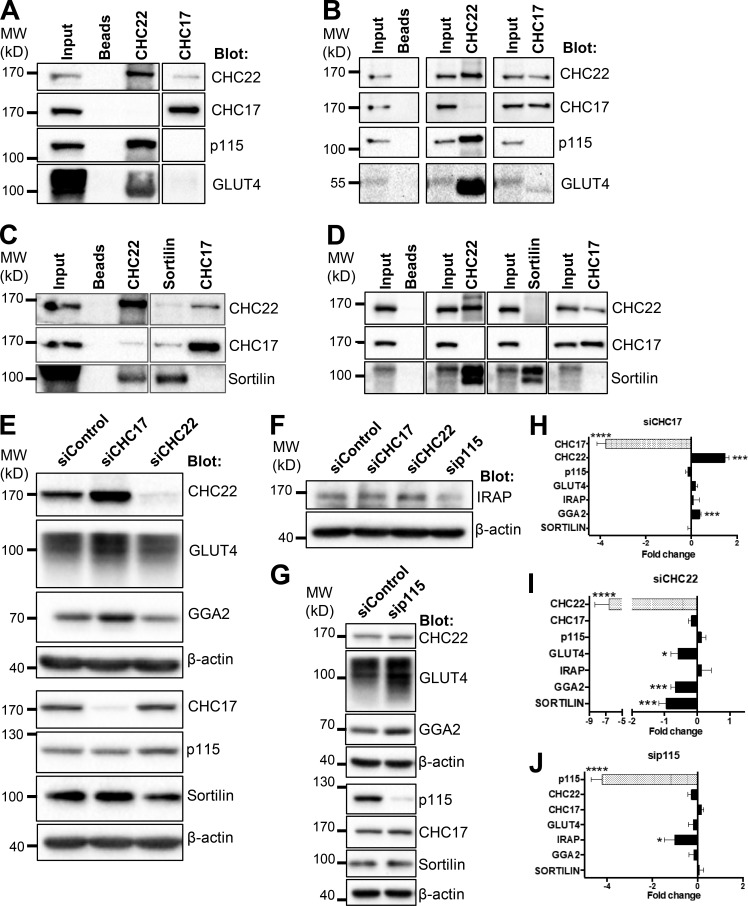
Figure 6.
CHC22 interacts with p115 and each influences stability of different partners involved in GLUT4 membrane traffic. (A–D) Representative immunoblots of immunoprecipitates of CHC22, CHC17 (A–D), or sortilin (C and D) from HeLa-GLUT4 cells (A and C) and hSKMC-AB1190 (B and D) immunoblotted for CHC22, CHC17, p115, GLUT4, and sortilin. The position of MW markers is indicated in kilodaltons at the left. (E–G) Representative immunoblots of HeLa-GLUT4 cells transfected with siRNA targeting CHC22, CHC17 (E and F), or p115 (F and G), or with nontargeting control siRNA (40 nM for 72 h) showing levels of CHC22, GLUT4, GGA2, CHC17, p115, sortilin, and β-actin (E and G) or levels of IRAP and β-actin (F). The position of MW markers is indicated in kilodaltons at the left. In HeLa-GLUT4 cells, GLUT4 is tagged, altering its migration compared with muscle cell GLUT4. (H–J) Quantifications of immunoblot signals as shown in E–G. Blot signals were normalized to β-actin for each experiment and the fold change (negative values indicate decrease and positive values indicate increase) relative to the normalized signal in control siRNA-treated cell lysates is plotted. Data expressed as mean ± SEM, n = 7–8. Two-tailed unpaired Student’s t test, with Welch’s correction where variances were unequal: *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.