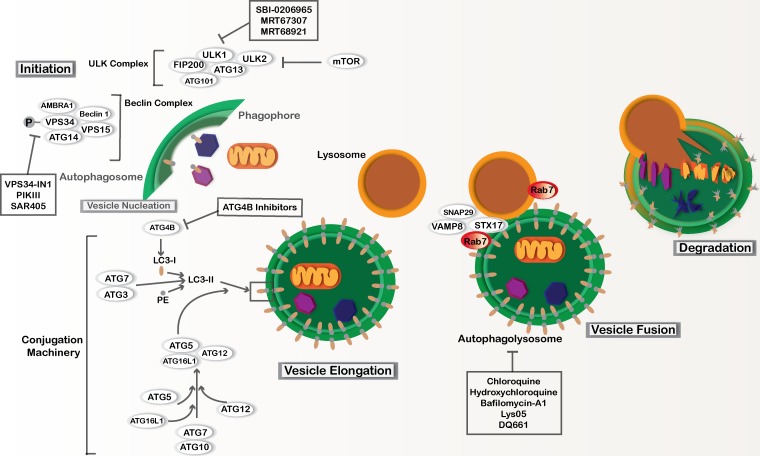
Figure 1.
Macro-autophagy. Macro-autophagy involves core autophagy proteins or ATGs and is subdivided into different stages, including phagophore initiation, vesicle nucleation, vesicle elongation, and autophagosome fusion with lysosomes. The ULK complex involves Unc-51–like autophagy activating kinases 1 and 2 (ULK1 and ULK2), ATG13, ATG101, and FAK family kinase interacting protein of 200 kD (FIP200). This complex can be regulated by nutrient availability via mTOR regulation as well as other signaling pathways to induce phagophore initiation. The Beclin complex is activated downstream of the ULK complex and is also necessary for phagophore initiation. The Beclin complex includes coiled-coil, moesin-like, BCL2 interacting protein (Beclin-1), activating molecule in Beclin-1 regulated autophagy (AMBRA-1), phosphatidylinositol 3-kinase catalytic subunit type 3 and regulatory subunit 4 (VPS34 and VPS15, respectively), and ATG14. Vesicle elongation depends on two ubiquitin-like conjugation systems. ATG5 is conjugated to ATG12 with the help of the E1-like enzyme, ATG7, and the E2-like enzyme, ATG10. The ATG5–ATG12 conjugate binds to ATG16L1, and together they act as a E3-like enzyme to facilitate the conjugation of microtubule-associated protein 1A/1B LC3 to PE. This second conjugation is also aided by ATG7 as well as the E1-like enzyme, ATG3. Prior to LC3-PE conjugation, LC3 is cleaved by the cysteine protease ATG4B. LC3-PE is incorporated into the autophagosome membrane. SNARE proteins including syntaxin-17 (STX17), synaptosome-associated protein 29 (SNAP29), and vesicle-associated membrane protein 8 (VAMP8) facilitate fusion between fully formed autophagosomes and lysosomes. The GTPase Rab7 is also important during fusion. After fusion occurs, the cytoplasmic material within the autolysosome as well as the intravesicular LC3-II is degraded by pH-sensitive enzymes found within the acidic compartments. Pharmacological agents that are currently used to inhibit autophagy in preclinical models are annotated.