Abstract
The regulation mechanisms involved in matrix metalloproteinase (MMP) expression and the motility of human endometrial and decidual stromal cells (ESCs and DSCs, respectively) during decidualization remain unclear. DSCs show significant increased cell motility and expression of FOS-like 1 (FOSL1) and MMP1, MMP2, and MMP9 compared with ESCs, whereas lack of decidualization inducers leads to a rapid decrease in FOSL1 and MMP1 and MMP9 expression in DSCs in vitro. Therefore, we hypothesized that a link exists between decidualization inducers and FOSL1 in up-regulation of motility during decidualization. Based on the response of ESCs/DSCs to different decidualization systems in vitro, we found that progesterone (P4) alone had no significant effect and that 17β-estradiol (E2) significantly increased cell motility and FOSL1 and MMP1 and MMP9 expression at the mRNA and protein levels, whereas 8-bromo-cAMP significantly decreased cell motility and FOSL1 and MMP9 expression in the presence of P4. In addition, we showed that E2 triggered phosphorylation of estrogen receptor 1 (ESR1), which could directly bind to the promoter of FOSL1 in ESCs/DSCs. Additionally, we also revealed silencing of ESR1 expression by siRNA abrogated E2-induced FOSL1 expression at the transcript and protein levels. Moreover, silencing of FOSL1 expression by siRNA was able to block E2-induced MMP1 and MMP9 expression and cell motility in ESCs/DSCs. Taken together, our data suggest that, in addition to its enhancement of secretory function, the change in MMP expression and cell motility is another component of the decidualization of ESCs/DSCs, including estrogen-dependent MMP1 and MMP9 expression mediated by E2–ESR1–FOSL1 signaling.
Keywords: cell migration, cell invasion, estrogen, estrogen receptor, matrix metalloproteinase (MMP), decidualization, FOS-like 1, human decidual stromal cells, human endometrial stromal cells
Introduction
Characterized by endometrial stromal cell (ESC)3 decidualization, vascular reconstruction, immune cell recruitment, and plentiful molecule production, decidualization of the endometrium plays an important role in successful pregnancy (1–3). Initiated by postovulatory changes in steroid hormones, decidualization of human ESCs continues after establishment of pregnancy, and ESCs are transformed into gestation-specific decidual stromal cells (DSCs). Decidualization transforms morphological and biochemical features, including increased cell volume, expanded cytoplasm, accumulation of glycogen and lipid droplets, and, most importantly, increased secretory function (1). Moreover, in addition to recognized decidualization products such as prolactin (PRL), ESCs and DSCs secrete various matrix metalloproteinases (MMPs) to degrade extracellular matrix components and activate growth factors, contributing to cyclical breakdown and regeneration of the endometrium as well as decidua remodeling (4, 5). DSCs in particular have been reported to be a rich source of MMPs at the human fetal–maternal interface, in addition to invasive trophoblasts and natural killer cells (6, 7). Studies have suggested that increased MMP expression is implicated in vasodilation, placentation, and uterine expansion during normal rat pregnancy, whereas decreased expression of MMPs has been associated with abnormal uteroplacental and vascular remodeling because of collagen deposition in hypertensive pregnancy (8, 9). Abnormal uterus MMP expression/activity in preeclampsia patients has been observed to be involved in the pathogenesis of preeclampsia (10–12). Moreover, production of MMPs indicates an intrinsically motile and invasive capacity of ESCs/DSCs, and previous reports have revealed the necessity of ESC motility for embryo implantation and encapsulation (13, 14). This evidence indicates the importance of MMP expression as well as its migration and invasion ability in ESCs/DSCs for reproductive health; however, its underlying regulation mechanisms have remained unclear. Active protein 1 (AP-1), a menagerie of dimeric–basic region–leucine zipper proteins that belong to the Jun and Fos subfamilies, is implicated as a regulator in cell proliferation, invasion, and differentiation and contributes to transactivation or repression of gene expression by binding to the promoters of its target genes in a sequence-specific manner (15, 16). In addition, some AP-1 members have been found to be expressed in the endometrium and placenta (17). Moreover, the promoters in the majority of human MMP genes contain an AP-1–binding site at approximately −70 bp, and AP-1 has been widely reported to regulate MMP expression in several cell types (18). A previous study has suggested that myometrial AP-1 drives the production of MMP3 and MMP10, resulting in preterm labor in mice (19). Therefore, AP-1 probably regulates MMP expression, migration, and invasion of human ESCs/DSCs. In this study, we found significantly increased MMP and FOS-like 1 (FOSL1, also known as FOS-related antigen 1 (FRA-1), a member of the Fos family) expression in DSCs compared with ESCs. Moreover, cultures without decidualization inducers experienced a rapid decrease in the FOSL1 and MMP1 and MMP9 expression in DSCs in vitro. Therefore, we hypothesized that a link exists between decidualization inducers and FOSL1 in dramatic up-regulation of MMP1 and MMP9 and cell motility. In this study, we explored whether FOSL1 mediates promotion of MMP1 and MMP9 expression and cell motility of ESCs/DSCs via decidualization inducers and potential molecular mechanisms.
Results
MMP1, MMP2, and MMP9 expression, migration, and invasion ability of ESCs/DSCs
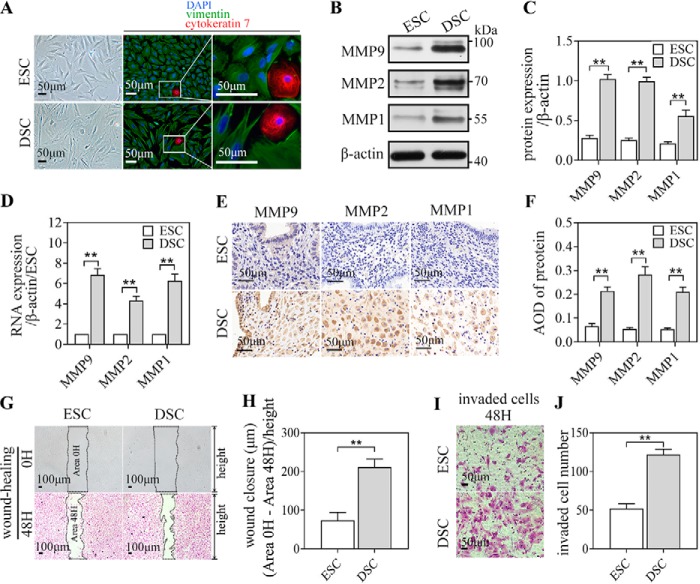
Primary ESCs isolated from the secretory endometrium and DSCs isolated from early-pregnancy decidua were identified as stromal cells based on their fusiform appearance, observed under an optical microscope, and their cell-specific expression, determined via immunofluorescence (IF) staining: vimentin-positive (mesenchyme origin–specific, green) and cytokeratin 7–negative (epithelial cell–specific, red) (Fig. 1A). Moreover, compared with ESCs, DSCs had a larger size with abundant cytoplasm, as seen under an optical microscope. DSCs also had significantly higher expression of MMP1, MMP2, and MMP9 than ESCs at both the protein and mRNA levels, as detected by western blot (WB) (Fig. 1, B and C), quantitative real-time PCR (qRT-PCR) of the isolated cells (Fig. 1D), and immunocytochemistry (IHC) of the endometrium/decidua (Fig. 1, E and F). To identify the migration and invasion ability of ESCs/DSCs, we employed a wound-healing and invasion assay in vitro. DSCs showed an approximate 3-fold increase in the length of wound closure (Fig. 1, G and H) and an approximate 2-fold increase in the number of invaded cells (Fig. 1, I and J) compared with ESCs.
Figure 1.
MMP1, MMP2, and MMP9 expression, migration, and invasion ability of ESCs/DSCs. A, primary stromal cells isolated from secretory endometrium (n = 3∼5/group) and early pregnancy decidua (n = 3∼5) were identified as stromal cells based on their fusiform appearance, observed via an optical microscope and immunofluorescence staining: vimentin-positive (mesenchyme origin–specific, green) and cytokeratin 7–negative (epithelial cell–specific, red) DAPI, 4,6-diamidino-2-phenylindole. These isolated cells were analyzed via Western blotting and quantitative real-time PCR to investigate the expression of MMP1, MMP2, and MMP9. B, and C, Western blot of ESCs/DSCs and its quantitative representation. D, relative mRNA expression. E and F, immunocytochemistry of the secretory endometrium and decidua against MMP1, MMP2, and MPP9 and quantitative representation of the average optical density (AOD) of each protein. Primary ESCs and DSCs were subject to wound healing and an invasion assay in vitro to explore their migration and invasion ability. G, photographs recorded at 0 and 48 h following application of the wound. The length of wound closure is equal to the difference in wound area divided by the height of the wound after converting to micrometers by scale. H, quantitative representation. I and J, the invaded cells in the invasion assay were recorded photographically after 48 h (I), and the quantitative representation of the number of invaded cells is shown (J). **, p < 0.01; error bars represent standard error of the mean. The data presented are from three independent experiments.
FOSL1 expression in ESCs/DSCs
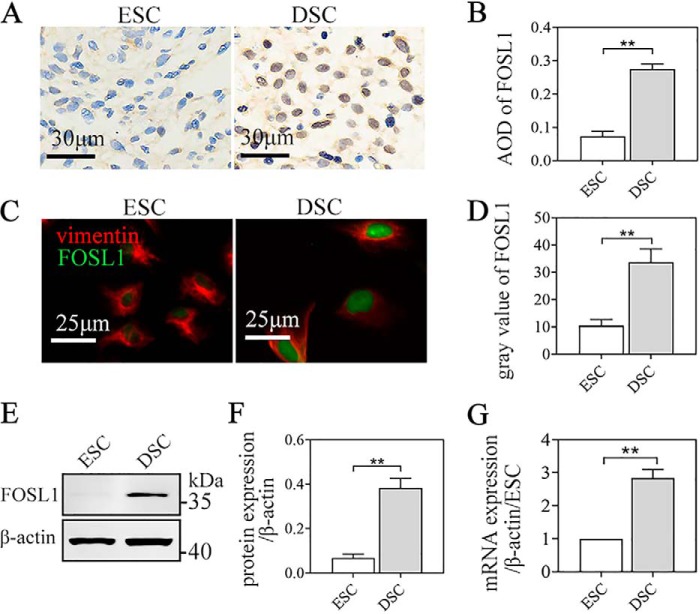
We found an ∼3-fold increase in the FOSL1 proteins of DSCs compared with ESCs, as investigated by IHC of the endometrium/decidua (Fig. 2, A and B) and IF (Fig. 2, C and D) and WB of the isolated cells (Fig. 2, E and F). Consistent with protein expression, we found significantly higher mRNA expression in DSCs compared with ESCs via qRT-PCR assay of the isolated cells (Fig. 2G).
Figure 2.
FOSL1 expression in ESCs/DSCs. The protein expression of FOSL1 in ESCs/DSCs was analyzed via immunocytochemistry staining of the secretory endometrium and early pregnancy decidua (n = 3∼5) as well as immunofluorescence and Western blotting of the isolated primary cells (n = 3∼5). A and B, immunocytochemistry against tissue FOSL1 and quantitative representation of the average optical density (AOD) of FOSL1. C and D, immunofluorescence of cells against FOSL1 and vimentin and quantitative representation of the average gray value of FOSL1. E and F, Western blot of cells and its quantitative representation. G, mRNA expression of FOSL1 in ESC/DSC was analyzed via quantitative real-time PCR of the isolated cells. **, p < 0.01; error bars represent standard error of the mean. The data presented are from three independent experiments.
FOSL1, MMP, PRL, and IGFBP1 expression, migration, and invasion ability of ESCs/DSCs during decidualization in vitro
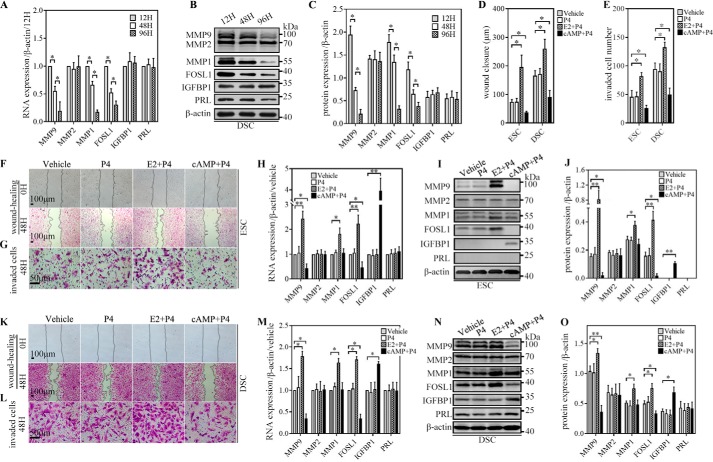
First, we observed the expression of FOSL1; MMP1, MMP2, and MMP9; PRL, and insulin-like growth factor-binding protein 1 (IGFBP1) in DSCs during 96-h culturing in vitro without a decidualization inducer after isolation from the decidua. Investigated via qRT-PCR and WB, FOSL1 and MMP1 and MMP9 rapidly decreased, whereas MMP2, PRL, and IGFBP1 remained stable at both the mRNA and protein levels (Fig. 3, A–C). Second, we investigated the response of ESCs/DSCs to different decidualization inducers in vitro. Primary ESCs/DSCs isolated from tissue were immediately subjected to three different decidualization systems in vitro for 48 h as follows: progesterone (P4, 1 μm), 17β-estradiol (E2, 0.1 μm) along with P4 (1 μm), and 8-Bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP, 0.5 mm) along with P4 (1 μm). A culture with a basic medium-containing vehicle served as the control. As illustrated by quantitative representation of the length of wound closure and the number of invaded cells (Fig. 3, D and E), both cell migration and invasion of ESCs/DSCs showed a significant increase induced by E2 + P4, a decrease induced by 8-Br-cAMP + P4, and no change by P4 alone compared with vehicle, as investigated via wound healing (Fig. 3, F and K) and invasion assays (Fig. 3, G and L) in vitro. Then we examined the expression of FOSL1; MMP1, MMP2, and MMP9; PRL, and IGFBP1 in ESCs/DSCs via qRT-PCR and WB. The results from qRT-PCR (Fig. 3, H and M) and WB and their quantitative representation (Fig. 3, I, J, N, and O) revealed that the expression of FOSL1 and MMP9 in ESCs/DSCs showed a consistent change with cell migration and invasion; their expression was increased by E2 + P4, decreased by 8-Br-cAMP + P4, and not affected by P4 compared with vehicle at both the mRNA and protein levels. However, only E2 + P4 significantly affected MMP1 expression of ESCs/DSCs and was seen as up-regulation at both mRNA and protein levels, whereas the other treatments did not affect its expression. Additionally, the decidualization systems did not affect MMP-2 expression of ESCs/DSCs compared with vehicle. PRL and IGFBP1, as two recognized decidual markers, are widely used to measure secretory function during decidualization. In all decidualization systems, only 8-Br-cAMP + P4 significantly induced/increased IGFBP1 expression of ESCs/DSCs after 48-h culture, and the other treatments failed to affect IGFBP1 and PRL expression of ESCs/DSCs after 48-h culture.
Figure 3.
FOSL1, MMP, PRL, and IGFBP1 expression, migration, and invasion ability of ESCs/DSCs during decidualization in vitro. Primary DSCs (n = 3∼5) were cultured with basic medium without decidualization inducers for 12, 48, and 96 h. Cells were harvested to investigate the expression of FOSL1; MMP1, MMP2, and MMP9; PRL, and IGFBP1 via quantitative real-time PCR and Western blotting. A, mRNA expression. B and C, protein expression and its quantitative representation. F, G, K, and L, primary ESCs (n = 3∼5) and DSCs were cultured or subjected to a wound-healing assay (F and K) and invasion assay (G and L) in three different decidualization systems in vitro for 48 h as follows: 1 μm P4, 0.1 μm E2 along with 1 μm P4, and 0.5 mm 8-Br-cAMP (cAMP) along with 1 μm P4. A basic medium–containing vehicle served as the control. D and E, quantitative representation of the length of wound closure and number of invaded cells. Expression of FOSL1; MMP1, MMP2, and MMP9; PRL, and IGFBP1 was investigated via quantitative real-time PCR and Western blotting. H and M, mRNA expression of ESCs (H) and DSCs (M). I, J, N, and O, Western blot of ESCs and DSCs (I and N, respectively) and their quantitative representation (J and O, respectively). *, p < 0.05; **, p < 0.01; error bars represent standard error of the mean. The data presented are from three independent experiments.
ESR1 mediated E2-induced FOSL1 expression in ESCs/DSCs in vitro
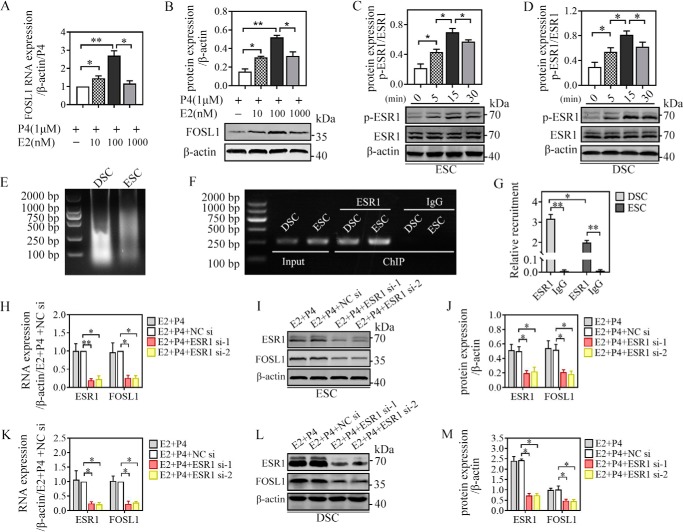
To investigate the regulatory role of E2 in regulating FOSL1 expression, we observed the expression profile of FOSL1 at different E2 concentrations (10, 100, and 1000 nm). The WB and qRT-PCR results indicated that the mRNA and protein expression of FOSL1 in ESCs was significantly promoted by E2 in a dose-dependent manner and that 0.1 μm was the optimal concentration (Fig. 4, A and B). As the predominant estrogenic acceptor in the human endometrium with high expression (17), estrogenic acceptor 1 (ESR1) may mediate estrogen-induced FOSL1 expression. First we detected the phosphorylated protein and total protein of ESR1 via WB in ESCs/DSCs stimulated by E2 after overnight starvation (Fig. 3, C and D). The WB results showed that p-ESR1/ESR1 levels were significantly increased by E2 and reached a peak 15 min after administration. We then considered the possibility that the FOSL1 gene is a direct target of ESR1 regulation during human ESC/DSC decidualization. The putative ESR1-binding site in the FOSL1 promoter was predicted by the JASPAR database, and ChIP analysis was performed to examine ESR1 occupancy at the candidate site in ESCs/DSCs after 48-h culture with E2 + P4. As expected, significant recruitment of ESR1 was seen for the FOSL1 promoter in ESCs and DSCs. In addition, DSCs showed significantly higher recruitment compared with ESCs (Fig. 4, F and G). Moreover, we verified mediation of ESR1 in E2-induced FOSL1 expression via ESR1 mRNA deletion. Knockdown of ESR1 was carried out via a 24-h pretreatment of ESR1 siRNA-1 and -2 transfection, and NC siRNA transfection served as the negative control. Then the cells were stimulated with 0.1 μm E2 in the presence of 1 μm P4 for 48 h to detect ESR1 and FOSL1 expression via qRT-PCR and WB. As shown in the results from qRT-PCR (Fig. 3, H and K) and WB (Fig. 3, I and L) and their quantitative representation (Fig. 3, J and M), ESCs/DSCs transfected with ESR1 siRNA-1 and -2 exhibited a remarkable decrease in ESR1 mRNA and protein expression, whereas transfection with NC siRNA did not affect ESR1 expression at the transcript and protein levels. Moreover, a decrease in ESR1 expression led to a consistent decline in FOSL1 expression in ESCs and DSCs. These findings indicated that ESR1 signaling mediated E2-induced FOSL1 expression in decidualizing stromal cells.
Figure 4.
ESR1 mediated E2-induced FOSL1 expression in ESCs/DSCs in vitro. A and B, primary ESCs (n = 3∼5) were cultured with different doses of 17β-estradiol (0, 10, 100, and 1000 nm) in the presence of 1 μm progesterone for 48 h, and the mRNA and protein levels of FOSL1 were analyzed via quantitative real-time PCR (A) and Western blotting (B), respectively. C and D, after overnight starvation with serum-free medium, primary ESCs and DSCs (n = 3∼5) were stimulated with serum-free medium containing 0.1 μm 17β-estradiol, and the total protein and phosphorylated protein of ESR1 were detected via Western blotting 0, 5, 15, and 30 min after administration. The possibility that the FOSL1 gene is a direct target of regulation by ESR1 during human ESC/DSC decidualization was investigated by ChIP. The putative ESR1-binding site in the FOSL1 promoter was predicted by the JASPAR database. ESR1 occupancy at the candidate site in ESCs/DSCs was detected after 48-h culture with 1 μm progesterone and 0.1 μm 17β-estradiol. E, the sonicated DNA fragments ranged from 200 bp to 1000 kb and were tested by agarose gel electrophoresis. PCR amplification formed the input control. F and G, ChIP with an ESR1 antibody and rabbit IgG were analyzed via agarose gel electrophoresis (F), and the relative recruitment was normalized to the signals obtained from the input DNA (G). Moreover, we verified mediation of ESR1 in estrogen-induced FOSL1 expression via ESR1 mRNA deletion. Expression of ESR1 mRNA was silenced by transfecting siRNA specific to human ESR1 for 24 h. Then cells were stimulated with 0.1 μm 17β-estradiol and 1 μm progesterone for 48 h to detect ESR1 and FOSL1 expression via quantitative real-time PCR and Western blotting. H and K, mRNA expression of ESCs (H) and DSCs (K). I, J, L, and M, Western blot of ESCs (I) and DSCs (L) and their quantitative representation (J and M, respectively). *, p < 0.05; **, p < 0.01; error bars represent standard error of the mean.
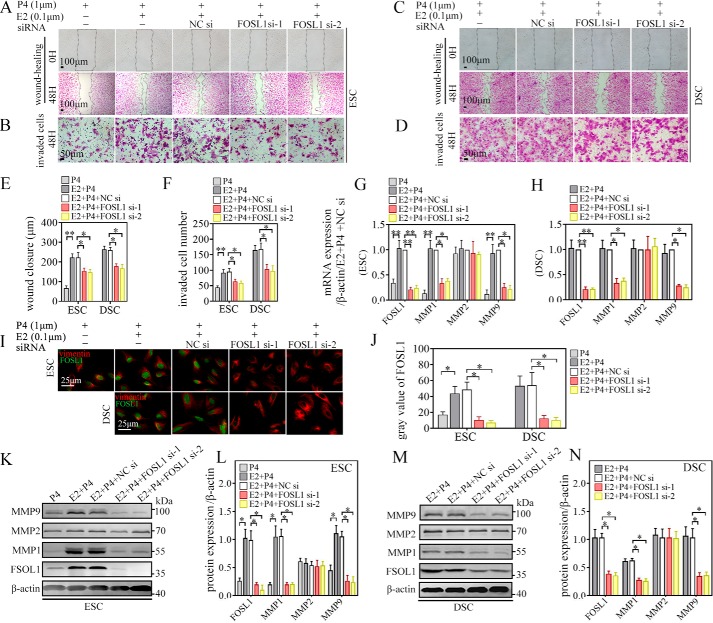
Silence of FOSL1 represses E2-induced MMP1 and MMP9 expression, migration, and invasion of ESCs/DSCs in vitro
Based on siRNA-mediated knockdown of mRNA expression, we next addressed the role of FOSL1 in E2-induecd MMP1 and MMP9 expression, migration, and invasion of ESCs/DSCs. ESCs were stimulated with 0.1 μm E2 along with 1 μm P4, and cells incubated with 1 μm P4 alone served as the control. Knockdown of FOSL1 was carried out with 24-h pretreatment of FOSL1 siRNA-1 and -2 transfection, and NC siRNA transfection served as the negative control. These treated cells were subjected to 48-h wound healing (Fig. 5A) and invasion assays (Fig. 5B) or harvested for mRNA and protein testing after 48-h culture. As shown in Fig. 5, E, and F, E2 + P4 significantly promoted cell migration and invasion of ESCs compared with P4 alone, as seen by the length of wound closure and number of invaded cells. Transfection with NC siRNA did not affect E2 + P4–induced increase in cell migration and invasion, whereas transfection with ESR1 siRNA-1 and -2 abrogated this increase. Similarly, as detected via qRT-PCR (Fig. 5G), IF (Fig. 5, I, top row, and J), and WB (Fig. 5, K and L), E2 + P4 stimulation led to a significant increase in MMP1 and MMP9 and FOSL1 expression at both the mRNA and protein levels in ESCs compared with P4 alone. Transfection with NC siRNA did not affect their expression, whereas transfection with ESR1 siRNA-1 and -2 inhibited E2-induced FOSL1 mRNA and protein expression, which was accompanied by a decrease in MMP1 and MMP9 expression at both the transcript and protein levels. As for DSCs, after 24-h transfection with FOSL1 siRNA-1 and -2 or NC siRNA, cells were subjected to 48-h wound healing (Fig. 5C) and invasion (Fig. 5D) assays or additional 48 h of culture in vitro with 0.1 μm E2 + 1 μm P4 to sustain decidualization. Similar to ESCs, FOSL1 siRNA-1 and -2 transfection significantly decreased the length of wound closure and the number of invaded cells (Fig. 5, E and F) as well as mRNA (Fig. 5H) and protein (Fig. 5, I, bottom row, J, M, and N) expression of FOSL1 and MMP1 and MMP9, whereas NC siRNA pretreatment did not cause significant changes in DSCs. However, mRNA and protein expression of MMP2 in ESCs/DSCs remained stable with all treatments. These results suggested that E2-induced MMP1 and MMP9 expression, migration, and invasion during decidualization depended on FOSL1.
Figure 5.
FOSL1 deletion represses E2-induced MMP1 and MMP9 expression, migration, and invasion ability of ESCs/DSCs in vitro. The role of FOSL1 in the estrogen-induced MMP1 and MMP9 expression, migration, and invasion of ESC/DSC was investigated by siRNA-mediated knockdown of mRNA expression. Primary ESCs (n = 3∼5) were stimulated with 0.1 μm 17β-estradiol along with 1 μm progesterone, and cells incubated with 1 μm progesterone alone served as the control. Primary DSCs (n = 3∼5) were incubated with 0.1 μm 17β-estradiol and 1 μm progesterone to sustain decidualization. Knockdown of FOSL1 was carried out with 24-h pretreatment of FOSL1 siRNA-1 and -2 transfection, and NC siRNA transfection served as the negative control. A–D, 48-h wound-healing and invasion assays of ESCs (A and B) and DSCs (C and D) were employed to explore migration and invasion ability, respectively. E and F, quantitative representations of the length of wound closure and invaded cell number. Expression of FOSL1 and MMP1, MMP2, and MMP9 was analyzed via quantitative real-time PCR, immunofluorescence, and Western blotting. G and H, relative RNA expression of ESCs and DSCs. I and J, immunofluorescence against FOSL1 of ESCs/DSCs and quantitative representation of the average gray value. K–N, Western blot of ESC/DSC and its quantitative representation. *, p < 0.05; **, p < 0.01; error bars represent standard error of the mean.
Discussion
The regulation mechanisms involved in MMP expression and the motility of ESCs/DSCs during decidualization remain unclear. Our work provided more evidence of the MMP expression profile and its promotion of migration and invasion in stromal cells during decidualization from the luteal phase to early pregnancy. We found significant up-regulation in MMP1, MMP2, and MMP9 expression, migration, and invasion of human DSCs compared with ESCs. These findings suggest that reorganization of MMP expression, migration, and invasion in stromal cells is an essential step in decidualization during early pregnancy, an important period for placenta formation.
By observing the MMP expression profile of DSCs during 96-h culture without a decidualization inducer after isolation from the decidua, we found a rapid decline in MMP1 and MMP9 expression, which may indicate dependence of MMP1 and MMP9 expression on a decidualization inducer. We found that P4 alone could not change both MMP expression and cell motility, as detected by different decidualization systems with the above inducer. In the presence of P4, E2 caused a significant increase in MMP1 and MMP9 expression, motility, and invasion of ESCs/DSCs, but 8-Br-cAMP + P4 induced a dramatic decrease in MMP9 expression, migration, and invasion of ESCs/DSCs. These findings were similar to those of a previous report on endometriosis (20); P4 was unable to stimulate migration of ESCs in healthy women, whereas E2 could stimulate motility of ESCs in both healthy women and endometriosis patients. Consistent with previous reports on the regulation of MMP expression by E2 in rodent/mice uteri (21–24), our findings indicate that estrogen signaling operates as a key mediator in MMP expression, migration, and invasion in ESCs/DSCs during decidualization rather than cAMP and progesterone signaling. Moreover, estrogen was necessary to induce or maintain the motility function of decidualization in ESCs/DSCs, and the system of E2 combined with P4 was suitable for an in vitro study of motility function during decidualization.
cAMP is a universal second messenger that plays an important role in intracellular signal transduction, including invasion, and exerts positive or negative regulation of MMP expression in different cell lines (25–27). Our findings provide new evidence of cAMP in down-regulation of MMP expression in ESCs/DSCs. We also found that cAMP led to a decrease in both MMP9 and FOSL1 in ESCs/DSCs, indicating that FOSL1 was probably involved in cAMP signaling. However, the decrease in FOSL1 expression in cAMP-treated cells did not affect expression of MMP1. This phenomenon reveals that the regulation of gene expression in ESCs/DSCs during decidualization is a comprehensive result of multiple factors, and much remains to be explored.
In addition, we found stable expression of PRL and IGFBP1 in DSCs during 96-h culture in vitro without an inducer. This stable PRL expression was consistent with a previous report of a human primary DSC culture without steroid hormones in vitro (28). PRL and IGFBP1 expression in different systems was similar to previous reports as well; E2 + P4 requires 7–10 days or more to induce PRL/GFBP1 secretion in human ESCs (29, 30), whereas cAMP + P4 requires 2 days to induce IGFBP1 secretion and 4 days to induce PRL secretion (31). These findings indicate that, compared with expression of PRL and IGFBP1, MMP1 and MMP9 expression of ESCs/DSCs have a faster response and higher dependence on the presence of E2.
As AP-1 family members, JUN, FOS, and FOSL1 have been widely reported to regulate MMP1 and MMP9 in several cell types (32–35). In particular, expression of JUN, FOS, and FOSL1 has been reported to increase by E2 (but not other AP-1 members) and decrease by antiestrogen tamoxifen to mediate MCF-7 cell proliferation (36). However, expression of JUN and FOS in human endometrium has been reported to be down-regulated during pregnancy (37). Therefore, we focused on observing FOSL1 expression in ESCs/DSCs. Similar to the MMP1 and MMP9 expression profiles, DSCs showed significant elevation in FOSL1 expression compared with ESCs and a dramatic decline in FOSL1 expression in vitro when cultured without a decidualization inducer. In addition, the FOSL1 expression of ESCs/DSCs showed a consistent change with migration and invasion in decidualization systems in vitro: increased by E2 and decreased by cAMP. Moreover, FOSL1 expression showed a significant increase induced by E2 in a dose-dependent manner. These findings indicate involvement of FOSL1 in regulation of E2 in terms of its migration and invasion in ESCs/DSCs. This hypothesis was verified in all subsequent experiments. Our results indicated that E2 triggered activation of ESR1, a predominant estrogenic acceptor in human endometrium/decidua (17), and that inhibition of ESR1 expression via siRNA attenuated E2-induced FOSL1 expression in ESCs/DSCs. Moreover, we also found that ESR1 specifically and strongly binds to the FOSL1 gene primer. This finding is similar to that of a previous study, which reported that ESR1 regulates FOSL1 expression by binding to the FOSL1 promoter in mouse uterine stroma (23). In addition, our work provides direct evidence of FOSL1 as a downstream target of ESR1 in human primary ESCs/DSCs. Via siRNA-mediated mRNA deletion, our work verified positive regulation of FOSL1 in MMP1 and MMP9 expression of ESCs/DSCs at the transcript level; E2-induced FOSL1 expression facilitated MMP1 and MMP9 expression at the transcript and protein levels, and migration, invasion, and deletion of FOSL1 expression by siRNA represses this promotion. FOSL1 has been shown to directly induce MMP1 and MMP9 promoter activity in human breast cancer cell lines (38) and occupy MMP9 promoter regions in trophoblast cells (39). Therefore, it is plausible that FOSL1 may function in a similar fashion in ESCs/DSCs. However, more studies are needed to validate this hypothesis.
Taken together, our data reveal a novel pathway mediated by reorganization of MMP1 and MMP9 expression, migration, and invasion in human ESCs/DSCs during decidualization, highlighting the key regulatory role of E2 and suggesting that FOSL1 expression induced by E2–ESR1 signaling may be a key transcription factor. Studies in animal models have found that release of bioactive factors induced by ischemic/hypoxic placentas, which target MMPs in the extracellular matrix, contribute to altered uteroplacental and vascular remodeling (8, 40–42). Therefore, MMPs and their meditators could be potential targets for drug development in pathological pregnancy (43). Our findings are of great significance in revealing that FOSL1, as an E2-induced decidualization meditator of ESCs/DSCs involved in MMP expression, migration, and invasion, may be a probable target and that E2, as a common obstetric and gynecological drug, may be administrated for treatment of embryo implantation failure and placental dysplasia because of its regulation of MMP expression, migration, and invasion of ESCs/DSCs. Future studies are needed to address the mechanisms by which FOSL1 regulates the MMPs of ESCs/DSCs as well as those by which MMPs, in turn, affect decidua remodeling, embryo implantation, and placental formation.
Experimental procedures
Reagents
Progesterone (dissolved with absolute ethanol), 17β-estradiol (dissolved with absolute ethanol), and 8-Br-cAMP (dissolved with 1× PBS) were from Sigma-Aldrich (St. Louis, MO). Antibodies against human MMP-1, MMP-2, and MMP-9; IGFBP1; cytokeratin 7; vimentin; and β-actin were from Cell Signaling Technology (Danvers, MA). Antibodies against human estrogen receptor 1 (ESR1), p-ESR1, FOSL1, and PRL were from Abcam (Cambridge, MA). All primers were from Sangon Biotech (Shanghai, China). FOSL1 and ESR1 siRNA was from Genepharma (Shanghai, China).
Tissue collection
Normal decidua samples were obtained from healthy women aged 20–35 years with a gestational age of 6–7 weeks (Department of Obstetrics and Gynecology, Renji Hospital). All patients underwent elective first-trimester pregnancy termination upon the patients' own request without medical reasons. Endometrial tissues were obtained from patients aged 20–46 years undergoing sterilization or hysterectomy for benign conditions. Patients had regular menstrual cycles and had received no hormonal medication in the past 3 months. Samples were obtained during the mid-secretory stage of the menstrual cycle, as confirmed by histological examination. All patients provided signed consent forms for tissue collection. This study was approved by the Human Research Ethics Committee of the School of Medicine of Shanghai Jiaotong University.
Isolation of human primary ESCs/DSCs
Human endometria and deciduae were collected under sterile conditions and immediately transported to the laboratory in ice-cold DMEM/F-12 (HyCloneTM, Invitrogen). The obtained deciduae were pooled, washed in Ca2+Mg2+-free 1× PBS, and minced into 1- to 2-mm pieces. The minced tissues were digested by 0.1% g/ml collagenase type II (Sigma) in a sterile centrifuge tube in a 37 °C water bath with gentle shaking for 15 min, followed by a repeated cycle of 15-min digestion. To remove the undigested tissue and epithelial cells, the cell suspension was successively filtered through sterile 100- and 200-mesh wire sieves for DSCs and more 400-mesh wire sieves for ESCs. The filtered suspension was centrifuged at 450 × g for 3 min. After the supernatant was discarded, the cell pellets were resuspended in DMEM/F-12 containing 10% FBS (Gibco), 100 IU/ml penicillin, and 100 mg/ml streptomycin at 37 °C in a humidified environment with 5% CO2. After 6 h in the culture, primary stromal cells adhered to the wall. The medium was changed, and the suspended cells, such as leukocytes and erythrocytes, were aspirated. Both ESCs and DSCs were ∼98% pure, as assessed by cytokeratin (negative) and vimentin (positive).
Decidualization in vitro
Three common decidualization systems (29, 31) were used in vitro as follows. For the P4 method, ESCs were cultured with basic medium containing 1 μm P4. For the E2 + P4 method, ESCs were cultured with basic medium containing 0.1 μm E2 and 1 μm P4. For the 8-Br-cAMP + P4 method, ESCs were cultured with basic medium containing 0.5 mm 8-Br-cAMP and 1 μm P4. Basic medium–containing vehicle served as a control. The basic medium was DMEM/F-12 containing 2% FBS, 100 IU/ml penicillin, and 100 mg/ml streptomycin.
Wound healing assay
Human stromal cells with or without pretreatment were seeded into 6-well plates at a density of 2 × 105 cells/well to ensure that the cells reached confluence by the next day. After 6 h, the medium was changed to new serum-free decidualization medium to starve the cells overnight. Confluent monolayer stromal cells were scratched with a 200-ml sterile pipette tip the next day. The cell debris was washed away with 1× PBS, and the remaining cells were cultured with fresh serum-free decidualization medium for an additional 48 h. Wound width was monitored by phase-contrast microscopy at regular intervals. The extent of cell migration was assessed by calculating wound closure. As shown in Figs. 1, G and I, wound closure was equal to the difference in wound area divided by the height of the wound after conversion to micrometers by scale.
Invasion assay
Human stromal cells with or without pretreatment were suspended in a free serum medium at a density of 5 × 104 cells/ml. A 200-μl cell suspension was placed into the upper chamber (8 μm, Corning) precoated with Corning® Matrigel® Matrix (Corning) diluted with DMEM/F-12 at 1:6. Then 600 μl of various decidualization media, supplemented with 2% FBS, were added to the lower chamber. After incubation for 48 h at 37 °C, the cells in the upper chambers were carefully removed. The cells that had invaded through the membrane were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The invaded cells were counted using an IX71 inverted microscope (Olympus, Tokyo, Japan) at ×200 magnification.
Western blotting
Whole-cell extracts were prepared from human primary stromal cell cultures undergoing different treatments. Briefly, the cells were washed with an ice-cold balanced solution and lysed with radioimmune precipitation assay buffer (Sigma-Aldrich) containing a protease inhibitor mixture, PMSF (0.1 mg/ml), and a phosphatase inhibitor (1:1000, Sigma-Aldrich) for 30 min on ice. This was followed by centrifugation at 12,000 rpm for 10 min to remove cell debris. 20–50 μg of the protein extract was analyzed via SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Sigma-Aldrich). The membrane was blocked with 5% albumin in Tris-buffered saline with 0.1% Tween 20 for 1 h at room temperature, followed by incubation with a primary antibody against FOSL1; p-FOSL1; ESR1; p-ESR1; PRL; IGFBP1; MMP1, MMP2, and MMP9 or β-actin. The blot was then incubated with the corresponding IRDye 800CW-conjugated secondary antibody for 60 min at room temperature. Signals were visualized using the Odyssey IR Imaging System (LI-COR Biosciences, Lincoln, NE).
Quantitative real-time PCR
RNA was extracted using the TaKaRa MiniBEST Universal RNA Extraction Kit (Takara, Otsu, Japan) according to the manufacturer's instructions. RNA concentrations were measured by NanoDrop ND-1000 (Thermo Scientific, Waltham, MA). mRNA was synthesized into complementary DNA via the PrimeScript RT Reagent Kit (Takara) according to the manufacturer's instructions. Then qRT-PCR was performed using QuantStudio test development software (Life Technologies) with PrimeScriptTM RT Master Mix (Perfect Real Time, Takara). The data were analyzed using the 2-ΔΔCT method. β-Actin was used for data normalization, and the results were expressed as -fold change of the control group. The sequences of the primers were as follows: FOSL1, CCGCTGCTGCTGCTACTCTTG (reverse) and GAACCTGAGGCACTGCACACC (forward); ESR1, GTCTGCCAGGTTGGTCAGTAAGC (reverse) and ATGGTCAGTGCCTTGTTGGATGC (forward); MMP1, TCCCAGTCACTTTCAGCCCA (reverse) and GTGTCTCACAGCTTCCCAGC (forward); MMP2, CACCACGGATCTGAGCGATGC (reverse) and GCCTCTCCTGACA TTGACCTTGG (forward); MMP9, AGGGCACTGCAGGATGTCATA (reverse) and ACGACGT CTTCCAGTACCGAG (forward); PRL, GTGGATGATTCGGCACTTCAGGAG (reverse) and GCAGATGGCTGATGAAGAGTCTCG (forward); IGFBP1, GTTGGTGACATGGAGAGCCTTCG (reverse) and AGCACGGAGATAACTGAGGAGGAG (forward). β-Actin was used as an endogenous reference gene.
Immunohistochemistry
Briefly, paraffin-embedded endometria and decidua were sectioned and mounted on slides. The sections were rehydrated and washed in 1× PBS for 20 min and then incubated in a blocking solution containing 10% normal goat serum for 30 min before overnight incubation with a primary antibody against human MMP1, MMP2, and MMP9 and FOSL1 at 4 °C. Immunostaining was performed using the Avidin-Biotin Kit for Rabbit Primary Antibodies (Invitrogen). Sections were counterstained with hematoxylin, mounted, and examined under a bright field. Brown deposits indicated sites of positive immunostaining. The average optical density of each protein was identified via Image J software.
Immunofluorescence
The cells were fixed in 4% paraformaldehyde for 15 min at room temperature. After being rinsed three times in 1× PBS for 5 min each, the cells were blocked and permeabilized in blocking buffer (1× PBS, 5% normal serum, and 0.3% Triton X-100) for 60 min at room temperature. Thereafter, the cells were incubated overnight with a primary antibody against human cytokeratin, vimentin, and FOSL1 diluted in an antibody dilution buffer (1× PBS, 2% BSA, and 0.3% Triton X-100) at 4 °C, followed by 60-min incubation with a fluorochrome-conjugated secondary antibody at room temperature. Finally, after 4′,6-diamidino-2-phenylindole staining, the stained cells were observed and photographed under an inverted microscope (Axio Vert.A1, Carl Zeiss, Jena, Germany). The fluorescence intensity of each protein was recorded via a microscope as a gray value in the digital photograph. The average gray value of the proteins was determined using ImageJ software.
ChIP
ChIP was performed using a commercial ChIP assay kit (Merck Millipore, Billerica, MA) according to the manufacturer's instructions. Briefly, primary stromal cells isolated from a tissue were cultured for 48 h with 0.1 μm E2 and 1 μm P4. Then the chromatin was cross-linked by adding 1% formaldehyde for 10 min at room temperature, followed by addition of 1 m glycine for 5 min. The cross-linked chromatin was sonicated to obtain DNA fragments ranging from 200 bp to 1000 kb. One-tenth of the sonicated lysates was taken out as the input control. Immunoprecipitation was performed overnight at 4 °C with the ESR1 antibody, and rabbit IgG was used as a nonspecific control. The immune complexes were further precipitated using protein G/A–agarose washed with buffers, followed by elution of the protein–DNA complexes. Reverse cross-linking was then performed at 65 °C for 4 h, and the DNA was purified using spin columns. Quantitative PCR was performed on these purified DNA samples. The putative ESR1-binding site in the FOSL1 promoter was predicted using the JASPAR database (44), and the sequences of the primers were as follows: forward, AGGCAGGAGAATCCCTTTAGC; reverse, CCCACAAAATAGCACGAAAGAA. The PCR amplification products were analyzed via agarose gel electrophoresis. Relative recruitment was normalized to the signals obtained from the input DNA.
siRNA transfection
siRNA specifically against ESR1 and FOSL1 and NC siRNA were transiently transfected into ESCs or DSCs using a RFectTM transfection reagent (Changzhou Bio-generating Biotechnology, Changzhou, Jiangsu, China) according to the manufacturer's instructions. Briefly, primary stromal cells were seeded into 6-well plates at a density of 2 × 105 cells/well and reached 80%–90% confluence overnight. Then the medium was changed to 2500 μl of antibiotic-free DMEM/F12 containing 2% FBS/well. The 10 μl of transfection reagent and 2 μl of siRNA (10 nm) were diluted in 250 μl of Opti-MEMTM medium (Sigma-Aldrich), and then a diluted transfection reagent was gently added to the diluted siRNA. After incubation for 15 min at room temperature, the 500 μl of siRNA–transfection reagent complex was added to the cell wells. After 24-h incubation, the cells were subject to wound healing and invasion assays with fresh decidualization medium to investigate the motile and invasion ability or cultured for an additional 48 h with fresh decidualization medium to extract their mRNA and protein for qRT-PCR and Western blotting. The sequences of the siRNAs involved were as follows. FOSL1 siRNA-1, 5′-CUAGCACAAUUUGCACUAATT-3′ (sense) and 5′-UUAGUGCAAAUUGUGCUAGTT-3′ (antisense); FOSL1 siRNA-2, 5-′-GCUCAUCGCAAGAGUAGCATT-3′ (sense) and 5-′-UGCUACUCUUGCGAUGAGCTT-3′ (antisense); ESR1 siRNA-1, 5-′-GGAGAAUGUUGAAACACAATT-3′ (sense) and 5′-UUGUGUUUCAACAUUCUCCTT-3′ (antisense); ESR1 siRNA-2, 5′-GGGCUCUACUUCAUCGCAUTT-3′ (sense) and 5′-AUGCGAUGAAGUAGAGCCCTT-3′.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY). All experiments were performed at least three times in independent trials. The results are expressed as mean ± S.E. for the three independent experiments. Statistical analysis was conducted by comparing the means of the control and experimental sets using one-way analysis of variance. A value of p < 0.05 was considered statistically significant.
Author contributions
C. C., C. L., and A. Z. conceptualization; C. C., C. L., X. K., and S. S. data curation; C. C. software; C. C. and F. G. methodology; C. C. writing-original draft; C. L. formal analysis; W. L., T. Y., and S. L. sample collection.
Acknowledgments
We thank the Shanghai Key Laboratory of Gynecologic Oncology and the Department of Obstetrics and Gynecology of Renji Hospital for participating in this study.
This work was supported by National Natural Science Foundation of China Grants 81671481 and 81871179. The authors declare that they have no conflicts of interest with the contents of this article.
- ESC
- endometrial stromal cell
- DSC
- decidual stromal cell
- PRL
- prolactin
- MMP
- matrix metalloproteinase
- WB
- Western blot
- 8-Br-cAMP
- 8-bromo-cAMP
- NC
- negative control.
References
- 1. Gellersen B., and Brosens J. J. (2014) Cyclic decidualization of the human endometrium in reproductive health and failure. Endocrine Rev. 35, 851–905 10.1210/er.2014-1045 [DOI] [PubMed] [Google Scholar]
- 2. Mori M., Bogdan A., Balassa T., Csabai T., and Szekeres-Bartho J. (2016) The decidua–the maternal bed embracing the embryo–maintains the pregnancy. Sem. Immunopathol. 38, 635–649 10.1007/s00281-016-0574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu H., Hou C. C., Luo L. F., Hu Y. J., and Yang W. X. (2014) Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene 551, 1–14 10.1016/j.gene.2014.08.047 [DOI] [PubMed] [Google Scholar]
- 4. Dong J. C., Dong H., Campana A., and Bischof P. (2002) Matrix metalloproteinases and their specific tissue inhibitors in menstruation. Reproduction 123, 621–631 10.1530/rep.0.1230621 [DOI] [PubMed] [Google Scholar]
- 5. Anacker J., Feix S., Kapp M., Bausch R., and Kammerer U. (2010) Expression pattern of matrix metalloproteinases (MMPs) in human decidua during pregnancy. J. Reprod. Immunol. 86, 79 10.1016/j.jri.2010.08.002 [DOI] [Google Scholar]
- 6. Anacker J., Segerer S. E., Hagemann C., Feix S., Kapp M., Bausch R., and Kämmerer U. (2011) Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol. Hum. Reprod. 17, 637–652 10.1093/molehr/gar033 [DOI] [PubMed] [Google Scholar]
- 7. Weiss A., Goldman S., and Shalev E. (2007) The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front. Biosci. 12, 649–659 10.2741/2089 [DOI] [PubMed] [Google Scholar]
- 8. Li W., Mata K. M., Mazzuca M. Q., and Khalil R. A. (2014) Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem. Pharmacol. 89, 370–385 10.1016/j.bcp.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W., Cui N., Mazzuca M. Q., Mata K. M., and Khalil R. A. (2017) Increased vascular and uteroplacental matrix metalloproteinase-1 and -7 levels and collagen type I deposition in hypertension in pregnancy: role of TNF-α. Am. J. Physiol. Heart Circ. Physiol. 313, H491–H507 10.1152/ajpheart.00207.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J., and Khalil R. A. (2017) Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 148, 87–165 10.1016/bs.pmbts.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espino Y Sosa S., Flores-Pliego A., Espejel-Nuñez A., Medina-Bastidas D., Vadillo-Ortega F., Zaga-Clavellina V., and Estrada-Gutierrez G. (2017) New insights into the role of matrix metalloproteinases in preeclampsia. Int. J. Mol. Sci. 18, E1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahay A. S., Jadhav A. T., Sundrani D. P., Wagh G. N., Mehendale S. S., and Joshi S. R. (2018) Matrix metalloproteinases-2 (MMP-2) and matrix metalloproteinases -9 (MMP-9) are differentially expressed in different regions of normal and preeclampsia placentae. J. Cell. Biochem. 119, 6657–6664 10.1002/jcb.26849 [DOI] [PubMed] [Google Scholar]
- 13. Grewal S., Carver J. G., Ridley A. J., and Mardon H. J. (2008) Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc. Natl. Acad. Sci. U.S.A. 105, 16189–16194 10.1073/pnas.0806219105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grewal S., Carver J., Ridley A. J., and Mardon H. J. (2010) Human endometrial stromal cell rho GTPases have opposing roles in regulating focal adhesion turnover and embryo invasion in vitro. Biol. Reprod. 83, 75–82 10.1095/biolreprod.109.080630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vesely P. W., Staber P. B., Hoefler G., and Kenner L. (2009) Translational regulation mechanisms of AP-1 proteins. Mutat. Res. 682, 7–12 10.1016/j.mrrev.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 16. Shaulian E., and Karin M. (2002) AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4, E131–E136 10.1038/ncb0502-e131 [DOI] [PubMed] [Google Scholar]
- 17. Fagerberg L., Hallström B. M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., Asplund A., Sjöstedt E., Lundberg E., Szigyarto C. A., Skogs M., et al. (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics 13, 397–406 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan C., and Boyd D. D. (2007) Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 211, 19–26 10.1002/jcp.20948 [DOI] [PubMed] [Google Scholar]
- 19. MacIntyre D. A., Lee Y. S., Migale R., Herbert B. R., Waddington S. N., Peebles D., Hagberg H., Johnson M. R., and Bennett P. R. (2014) Activator protein 1 is a key terminal mediator of inflammation-induced preterm labor in mice. FASEB J. 28, 2358–2368 10.1096/fj.13-247783 [DOI] [PubMed] [Google Scholar]
- 20. Gentilini D., Vigano P., Somigliana E., Vicentini L. M., Vignali M., Busacca M., and Di Blasio A. M. (2010) Endometrial stromal cells from women with endometriosis reveal peculiar migratory behavior in response to ovarian steroids. Fertil. Steril. 93, 706–715 10.1016/j.fertnstert.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 21. Russo L. A., Peano B. J., Trivedi S. P., Cavalcanto T. D., Olenchock B. A., Caruso J. A., Smolock A. R., Vishnevsky O., and Gardner R. M. (2009) Regulated expression of matrix metalloproteinases, inflammatory mediators, and endometrial matrix remodeling by 17β-estradiol in the immature rat uterus. Reprod. Biol. Endocrinol. 7, 124 10.1186/1477-7827-7-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X., Christenson L. K., and Nothnick W. B. (2007) Regulation of MMP-9 expression and activity in the mouse uterus by estrogen. Mol. Reprod. Dev. 74, 321–331 10.1002/mrd.20582 [DOI] [PubMed] [Google Scholar]
- 23. Das A., Li Q., L., Laws M. J., Kaya H., Bagchi M. K., and Bagchi I. C. (2012) Estrogen-induced expression of Fos-related antigen 1 (FRA-1) regulates uterine stromal differentiation and remodeling. J. Biol. Chem. 287, 19622–19630 10.1074/jbc.M111.297663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lekontseva O., Jiang Y., and Davidge S. T. (2009) Estrogen replacement increases matrix metalloproteinase contribution to vasoconstriction in a rat model of menopause. J. Hypertens. 27, 1602–1608 10.1097/HJH.0b013e32832c41b5 [DOI] [PubMed] [Google Scholar]
- 25. Zhao P., Li X. G., Yang M., Shao Q., Wang D., Liu S., Song H., Song B., Zhang Y., and Qu X. (2008) Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol. Immunol. 45, 2187–2195 10.1016/j.molimm.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 26. Staun-Ram E., Goldman S., and Shalev E. (2009) Ets-2 and p53 mediate cAMP-induced MMP-2 expression, activity and trophoblast invasion. Reprod. Biol. Endocrinol. 7, 135 10.1186/1477-7827-7-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo D., Kassiri Z., Basu R., Chow F. L., Kandalam V., Damilano F., Liang W., Izumo S., Hirsch E., Penninger J. M., Backx P. H., and Oudit G. Y. (2010) Loss of PI3Kγ enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ. Res. 107, 1275–1289 10.1161/CIRCRESAHA.110.229054 [DOI] [PubMed] [Google Scholar]
- 28. Hamaguchi M., Yamamoto T., and Sugiyama Y. (1990) Production of prolactin by cultures of isolated cells from human first-trimester decidua. Obstet. Gynecol. 76, 783–787 10.1097/00006250-199011000-00011 [DOI] [PubMed] [Google Scholar]
- 29. Irwin J. C., Kirk D., King R. J., Quigley M. M., and Gwatkin R. B. (1989) Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil. Steril. 52, 761–768 10.1016/S0015-0282(16)61028-2 [DOI] [PubMed] [Google Scholar]
- 30. Hess A. P., Hamilton A. E., Talbi S., Dosiou C., Nyegaard M., Nayak N., Genbecev-Krtolica O., Mavrogianis P., Ferrer K., Kruessel J., Fazleabas A. T., Fisher S. J., and Giudice L. C. (2007) Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol. Reprod. 76, 102–117 10.1095/biolreprod.106.054791 [DOI] [PubMed] [Google Scholar]
- 31. Cloke B., Huhtinen K., Fusi L., Kajihara T., Yliheikkilä M., Ho K. K., Teklenburg G., Lavery S., Jones M. C., Trew G., Kim J. J., Lam E. W., Cartwright J. E., Poutanen M., and Brosens J. J. (2008) The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149, 4462–4474 10.1210/en.2008-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song Y., Qian L., Song S., Chen L., Zhang Y., Yuan G., Zhang H., Xia Q., Hu M., Yu M., Shi M., Jiang Z., and Guo N. (2008) Fra-1 and STAT3 synergistically regulate activation of human MMP-9 gene. Mol. Immunol. 45, 137–143 10.1016/j.molimm.2007.04.031 [DOI] [PubMed] [Google Scholar]
- 33. Chandrasekar B., Mummidi S., Mahimainathan L., Patel D. N., Bailey S. R., Imam S. Z., Greene W. C., and Valente A. J. (2006) Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-κB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J. Biol. Chem. 281, 15099–15109 10.1074/jbc.M600200200 [DOI] [PubMed] [Google Scholar]
- 34. Ray A., Shakya A., and Ray B. K. (2005) Inflammation-responsive transcription factors SAF-1 and c-Jun/c-Fos promote canine MMP-1 gene expression. Biochim. Biophys. Acta 1732, 53–61 10.1016/j.bbaexp.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 35. Kimura R., Ishikawa C., Rokkaku T., Janknecht R., and Mori N. (2011) Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim. Biophys. Acta 1813, 1543–1553 10.1016/j.bbamcr.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 36. Babu R. L., Naveen Kumar M., Patil R. H., Devaraju K. S., Ramesh G. T., and Sharma S. C. (2013) Effect of estrogen and tamoxifen on the expression pattern of AP-1 factors in MCF-7 cells: role of c-Jun, c-Fos, and Fra-1 in cell cycle regulation. Mol. Cell. Biochem. 380, 143–151 10.1007/s11010-013-1667-x [DOI] [PubMed] [Google Scholar]
- 37. Salmi A., Ammälä M., and Rutanen E. M. (1996) Proto-oncogenes c-jun and c-fos are down-regulated in human endometrium during pregnancy: relationship to oestrogen receptor status. Mol. Hum. Reprod. 2, 979–984 10.1093/molehr/2.12.979 [DOI] [PubMed] [Google Scholar]
- 38. Belguise K., Kersual N., Galtier F., and Chalbos D. (2005) FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 24, 1434–1444 10.1038/sj.onc.1208312 [DOI] [PubMed] [Google Scholar]
- 39. Kent L. N., Rumi M. A., Kubota K., Lee D. S., and Soares M. J. (2011) FOSL1 is integral to establishing the maternal-fetal interface. Mol. Cell. Biol. 31, 4801–4813 10.1128/MCB.05780-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yinon Y., Ben Meir E., Margolis L., Lipitz S., Schiff E., Mazaki-Tovi S., and Simchen M. (2015) Low molecular weight heparin therapy during pregnancy is associated with elevated circulatory levels of placental growth factor. Placenta 36, 121–124 10.1016/j.placenta.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 41. Shah D. A., and Khalil R. A. (2015) Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem. Pharmacol. 95, 211–226 10.1016/j.bcp.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ali S. M., and Khalil R. A. (2015) Genetic, immune, and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert Opin. Ther. Targets 19, 1495–1515 10.1517/14728222.2015.1067684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palei A. C., Granger J. P., and Tanus-Santos J. E. (2013) Matrix metalloproteinases as drug targets in preeclampsia. Curr. Drug Targets 14, 325–334 10.2174/1389450111314030004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathelier A., Fornes O., Arenillas D. J., Chen C. Y., Denay G., Lee J., Shi W., Shyr C., Tan G., Worsley-Hunt R., Zhang A. W., Parcy F., Lenhard B., Sandelin A., and Wasserman W. W. (2016) JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 44, D110–D115 [DOI] [PMC free article] [PubMed] [Google Scholar]