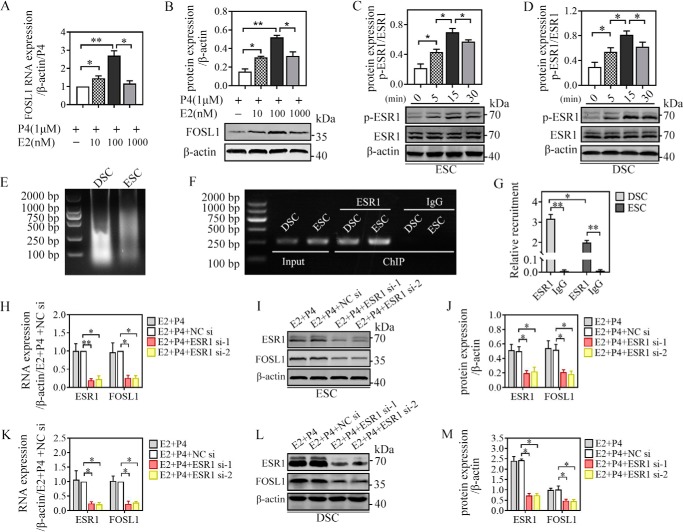
Figure 4.
ESR1 mediated E2-induced FOSL1 expression in ESCs/DSCs in vitro. A and B, primary ESCs (n = 3∼5) were cultured with different doses of 17β-estradiol (0, 10, 100, and 1000 nm) in the presence of 1 μm progesterone for 48 h, and the mRNA and protein levels of FOSL1 were analyzed via quantitative real-time PCR (A) and Western blotting (B), respectively. C and D, after overnight starvation with serum-free medium, primary ESCs and DSCs (n = 3∼5) were stimulated with serum-free medium containing 0.1 μm 17β-estradiol, and the total protein and phosphorylated protein of ESR1 were detected via Western blotting 0, 5, 15, and 30 min after administration. The possibility that the FOSL1 gene is a direct target of regulation by ESR1 during human ESC/DSC decidualization was investigated by ChIP. The putative ESR1-binding site in the FOSL1 promoter was predicted by the JASPAR database. ESR1 occupancy at the candidate site in ESCs/DSCs was detected after 48-h culture with 1 μm progesterone and 0.1 μm 17β-estradiol. E, the sonicated DNA fragments ranged from 200 bp to 1000 kb and were tested by agarose gel electrophoresis. PCR amplification formed the input control. F and G, ChIP with an ESR1 antibody and rabbit IgG were analyzed via agarose gel electrophoresis (F), and the relative recruitment was normalized to the signals obtained from the input DNA (G). Moreover, we verified mediation of ESR1 in estrogen-induced FOSL1 expression via ESR1 mRNA deletion. Expression of ESR1 mRNA was silenced by transfecting siRNA specific to human ESR1 for 24 h. Then cells were stimulated with 0.1 μm 17β-estradiol and 1 μm progesterone for 48 h to detect ESR1 and FOSL1 expression via quantitative real-time PCR and Western blotting. H and K, mRNA expression of ESCs (H) and DSCs (K). I, J, L, and M, Western blot of ESCs (I) and DSCs (L) and their quantitative representation (J and M, respectively). *, p < 0.05; **, p < 0.01; error bars represent standard error of the mean.