Abstract
Objective
To examine the effects of dynamic change in fetuin-A levels before the diagnosis of gestational diabetes mellitus (GDM) on insulin resistance and GDM.
Research design and methods
A total of 135 women with GDM and 135 normal glucose tolerance (NGT) women with matched age (±2 years old) and gestational age at taking the oral glucose tolerance test (OGTT) were included in this nested case–control study. Fasting venous blood samples were collected at the prenatal visit of the first trimester and during OGTT of the second trimester. Plasma concentration of fetuin-A and insulin was determined.
Results
The plasma fetuin-A concentration in women with GDM was significantly higher than NGT controls in both the first trimester (medians: 403.0 pg/mL vs 273.4 pg/mL; p<0.05) and the second trimester (medians: 475.7 pg/mL vs 290.8 pg/mL; p<0.05) and notably increased from the first to the second trimester. Multivariate linear regression analysis showed that the change in fetuin-A concentration was associated with the changes in fasting insulin, homeostasis model assessment (HOMA) of insulin resistance, and HOMA of β-cell function (HOMA-β) (p<0.05). The highest quartile of the increase in fetuin-A concentration from the first to the second trimester was associated with a higher risk of developing GDM compared with the lowest quartile (OR 2.14; 95% CI 1.05 to 4.37).
Conclusions
The dynamic change in fetuin-A levels was associated with the changes in insulin resistance and β-cell function from the first to the second trimester, and was associated with an increased risk of the development of GDM, indicating that fetuin-A could be a biomarker to predict the risk of GDM.
Trial registration number
Keywords: gestational diabetes mellitus, fetuin, insulin resistance
Significance of this study.
What is already known about this subject?
Fetuin-A has been shown to contribute to the onset of type 2 diabetes mellitus, indicating that it might also be an important biomarker involved in the pathogenesis of gestational diabetes mellitus (GDM) development. However, so far, limited research on the correlation of fetuin-A and GDM yielded inconsistent findings and none of them assessed the impact of the dynamic change in fetuin-A levels on GDM.
What are the new findings?
Plasma fetuin-A concentration in GDM cases was significantly higher than controls in both the first trimester and the second trimester and notably increased from the first to the second trimester.
The dynamic change in fetuin-A levels was associated with the changes in insulin resistance and β-cell function from the first to the second trimester, and was associated with an increased risk of the development of GDM.
How might these results change the focus of research or clinical practice?
The current findings indicated that fetuin-A may contribute to the pathogenesis of GDM and could be a biomarker to predict the risk of GDM. Further studies are needed to explore possible underlying mechanisms, and intervention studies could be carried out to assess if the reduction in fetuin-A concentration could lower the risk of GDM.
Introduction
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy.1 It was estimated that one in seven live births to women was affected by GDM worldwide in 2017.2 In China, the prevalence of GDM was 14.8%.3 GDM could cause serious short and long-term health risks for the mothers and offspring,4 which underpins the importance of early detection and management of GDM.
Fetuin-A, also known as α2-Heremans-Schmid glycoprotein, is a 64 kDa glycoprotein that is mainly synthesized and secreted from the liver and adipose tissue.5 Both animal and human studies have found that fetuin-A could inhibit insulin receptor tyrosine kinase activity and abolish the downstream signal cascades, resulting in insulin resistance and the onset of type 2 diabetes mellitus (T2DM).6–8 A recent meta-analysis of pooled results from seven prospective studies reported a 23% greater risk of incident T2DM per SD increment of fetuin-A level.9 Considering GDM shared the similar pathophysiological feature of insulin resistance with T2DM, and women with GDM were at high risk of developing T2DM in later life,10 it could be speculated that fetuin-A might also be an important biomarker involved in the pathogenesis of GDM development. However, so far, limited research on the correlation between fetuin-A and GDM yielded inconsistent findings.11–16 Some of them found significant differences in fetuin-A concentration between healthy pregnant and GDM group,12–14 while others did not.11 15 16 Previous studies were mainly limited by relatively small sample size and most of them measured fetuin-A concentration at or after the time of diagnosis of GDM. Only one paper focused on fetuin-A levels at a single time point before the diagnosis of GDM.14 None of the previous studies analyzed the dynamic change of fetuin-A concentration before the diagnosis of GDM. Thus, in this nested case–control study, we aimed to investigate the effects of dynamic change in fetuin-A levels before the diagnosis of GDM on insulin resistance and GDM.
Research design and methods
Study design
We performed a nested case–control study based on the Peking University Birth Cohort in Tongzhou (PKUBC-T). This cohort was registered in ClinicalTrials.gov. The PKUBC-T is a prospective cohort study carried out in Tongzhou District of Beijing, China, from June 2018 with primary aims to investigate the short-term and long-term health effects of pre-pregnant and prenatal exposures on mothers and their children from birth to 6 years old. Pregnant women having the first prenatal visit in Tongzhou Maternal and Child Health Hospital were included in the PKUBC-T with the following criteria: (1) age between 18 and 45 years old; (2) <14 gestational weeks; (3) resided in Tongzhou during the past half year and have no plan to move out after delivery; and (4) plan to have antenatal care and delivery in Tongzhou Maternal and Child Health Hospital. Women with pre-pregnancy diabetes, cardiovascular diseases, liver diseases, kidney diseases, and autoimmune diseases were excluded. All the included women were informed of the study protocol, and their written informed consents were obtained at the first prenatal visit.
Sample selection for the nested case–control study
Altogether, 5477 pregnant women met the inclusion criteria and were included in this cohort. By February 2019, a total of 3304 women have finished oral glucose tolerance test (OGTT) and 593 of them were diagnosed with GDM (17.9%). After excluding women with history of GDM, family history of diabetes, polycystic ovary syndrome, thyroid diseases, cigarette smoking, and alcohol consumption, 135 women with GDM were randomly selected and assigned to the case group, and 135 normal glucose tolerance (NGT) women with matched age (±2 years old) and gestation age at taking OGTT were assigned to the control group. Gestational age was defined as follows: In the first prenatal visit, pregnant women were interviewed by trained nurses to collect the date of their last menstrual period (LMP). If they had irregular menstrual cycles, the obstetricians would confirm their LMP based on the measurements from the ultrasound examinations. Gestational age at taking OGTT was calculated as the date of taking OGTT minus the date of LMP.
Data collection
At the first prenatal visit (7–13 gestational weeks), maternal characteristics, including demographic data, socioeconomic information, pre-pregnancy weights, pregnancy history, family history of diabetes, dietary intake, and physical activity of pregnant women in the prospective cohort, were collected. Pre-pregnancy weights were self-reported and collected by questionnaire at the first prenatal visit. Dietary intake was assessed using 24-hour dietary recall for 2 inconsecutive days and daily intake of calories was calculated. Physical activity was determined using the last 7 days, short form of the International Physical Activity Questionnaire,17 and quantified using metabolic equivalents of task (MET-min/week). Their heights, weights, and blood pressure were measured by trained nurses. Heights and weights were measured twice to ±0.5 cm and to ±0.1 kg, respectively, with subjects being barefoot and in light clothing. The averages of the measurements were recorded. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters. Gestational weight gain (GWG) before the diagnosis of GDM was calculated as weight measured at or before the diagnosis of GDM minus pre-pregnancy weight.
Biological sample collection and laboratory measurement
Venous blood samples of all participants were collected at the first prenatal visit after fasting overnight, centrifuged at 2500 relative centrifugal force for 10 min. Plasma was extracted and tested for fasting plasma glucose and biochemical indicators, including triglyceride, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatinine, alanine transaminase (ALT), and aspartate aminotransferase (AST), using standard detection methods. Glomerular filtration rate (GFR) was estimated using the modified Modification of Diet in Renal Disease (MDRD) equation for the Chinese population.18 The remaining fasting plasma collected at the first prenatal visit (7–13 gestational weeks) and during OGTT (25–28 gestational weeks) was aliquoted and stored at −80℃ refrigerator until assayed. The concentrations of fetuin-A and insulin in the plasma were batch analyzed in duplicate using the ELISA (R&D Systems China, Shanghai).
Outcome measures
The primary outcomes were insulin resistance and the development of GDM. Homeostasis model assessment (HOMA) was used to determine insulin resistance [HOMA-IR=insulin/(22.5e-ln glucose)], and β-cell function [HOMA-β(%)=20×insulin/(glucose-3.5)].19 GDM was diagnosed by OGTT. Between 24 and 28 gestational weeks, participants underwent a routine 75 g OGTT. Women with fasting glucose value ≥5.1 mmol/L, 1-hour postglucose ≥10.0 mmol/L or 2-hour postglucose ≥8.5 mmol/L were diagnosed with GDM.20
Statistical analyses
The distribution of continuous data was assessed with the Shapiro-Wilk test of normality. Variables that are not normally distributed are expressed as median (IQRs). Differences between women with GDM and controls were assessed by the Wilcoxon rank-sum test for non-parametric data. Longitudinal changes in fetuin-A, fasting insulin, HOMA-IR and HOMA-β from the first to the second trimester were analyzed by Wilcoxon signed-rank test. Multivariate linear regression analysis was performed to evaluate the independent associations of fetuin-A levels with fasting insulin, HOMA-IR and HOMA-β. Then, multivariate logistic regression models were used to estimate adjusted ORs of GDM in relation to fetuin-A quartiles in the first trimester, second trimester, and its change from the first to the second trimester, after adjustment for maternal age, gestational age at enrollment, pre-pregnancy BMI, GWG before the diagnosis of GDM, systolic blood pressure (SBP), diastolic blood pressure (DBP), GFR, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, ALT and AST, daily intake of calories, and weekly physical activity time. Besides, receiver operating characteristic (ROC) curves were used to test the predicted accuracy of fetuin-A concentration in the first trimester to diagnose GDM, and the diagnostic power was reported as area under the curve (AUC). SAS V.9.4 software was used to carry out all analyses and a two-sided value of p<0.05 was considered as statistically significant.
Results
Table 1 shows the baseline characteristics of women with GDM and controls. There were no significant differences in terms of age, gestational age at enrollment, pre-pregnancy BMI, GWG before the diagnosis of GDM, SBP, DBP, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, GFR, ALT, AST, daily intake of calories, and weekly physical activity time.
Table 1.
Baseline characteristics of participants in the first trimester
| Characteristic | GDM n=135 |
NGT n=135 |
P value |
| Age (years) | 29 (28–33) | 29 (28–33) | 1.00 |
| Gestational age at enrollment | 10 (9–12) | 10 (9–12) | 0.36 |
| Pre-pregnancy BMI (kg/m2) | 22.2 (20.3–25.1) | 22.0 (19.9–24.8) | 0.45 |
| GWG before OGTT (kg) | 8.6 (6.2–11.0) | 8.6 (6.4–10.5) | 0.56 |
| Systolic blood pressure (mm Hg) | 108 (101–116) | 110 (102–119) | 0.43 |
| Diastolic blood pressure (mm Hg) | 67 (62–73) | 67 (61–72) | 0.66 |
| Total cholesterol (mmol/L) | 4.0 (3.6–4.4) | 3.9 (3.6–4.4) | 0.84 |
| Triglyceride (mmol/L) | 1.2 (0.9–1.4) | 1.0 (0.9–1.3) | 0.07 |
| HDL cholesterol (mmol/L) | 1.7 (1.4–1.9) | 1.8 (1.5–1.9) | 0.26 |
| LDL cholesterol (mmol/L) | 2.2 (1.9–2.7) | 2.3 (1.9–2.6) | 0.79 |
| GFR (mL/min/1.73 m2) | 168.6 (151.9–182.6) | 172.6 (155.1–191.1) | 0.28 |
| ALT (U/L) | 13 (10–23) | 12 (10–19) | 0.33 |
| AST (U/L) | 14 (13–18) | 15 (13–17) | 0.92 |
| Daily intake of calories (kcal/day) | 1272 (1031–1631) | 1242 (936–1683) | 0.70 |
| Weekly PA time (MET-min/week) | 693 (238–1386) | 693 (198–1386) | 0.80 |
Data were presented as medians (IQR).
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; GDM, gestational diabetes mellitus; GFR, glomerular filtration rate; GWG, gestational weight gain; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalent of task; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; PA, physical activity.
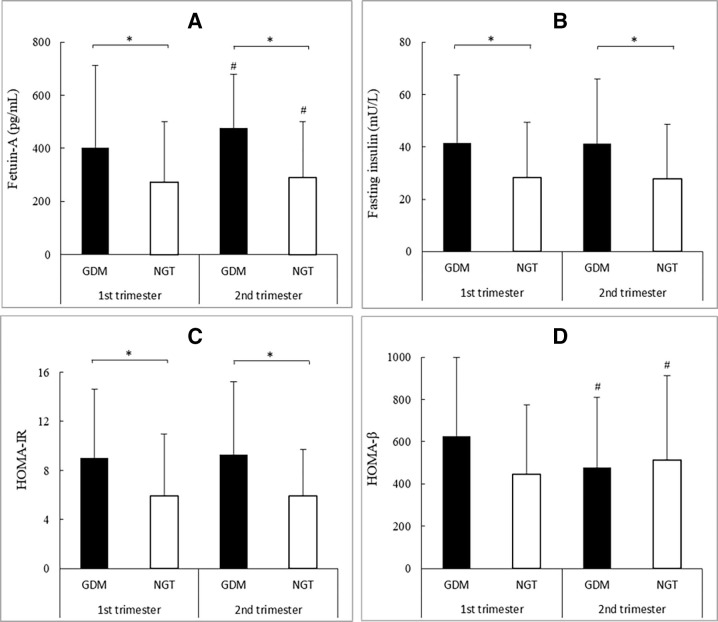
As shown in figure 1, the concentration of plasma fetuin-A in women with GDM was significantly higher than controls in both the first trimester (medians: 403.0 pg/mL vs 273.4 pg/mL; p<0.05) and the second trimester (medians: 475.7 pg/mL vs 290.8 pg/mL; p<0.05). From the first to the second trimester, the concentration of plasma fetuin-A increased significantly in both case group (medians: 403.0 pg/mL vs 475.7 pg/mL; p<0.05) and control group (medians: 273.4 pg/mL vs 290.8 pg/mL; p<0.05). HOMA-IR and the concentration of fasting insulin in the case group were significantly higher than in the control group in both the first and the second trimesters (all p<0.05). From the first to the second trimester, there was a progressive decrease in HOMA-β in the case group, while HOMA-β increased in the control group.
Figure 1.
Fetuin-A (A), fasting insulin (B), HOMA-IR (C), and HOMA-β (D) levels in the first and the second trimesters. Data were presented as medians (IQR). *P< 0.05. #P<0.05 versus first trimester in the same group. GDM, gestational diabetes mellitus; HOMA-IR, homeostasis model assessment of insulin resistance; NGT, normal glucose tolerance.
Multivariable linear regression analysis revealed that the change in fetuin-A from the first to the second trimester was associated with the changes in fasting insulin, HOMA-IR, and HOMA-β from the first to the second trimester, after adjustment for maternal age, gestational age at enrollment, pre-pregnancy BMI, GWG before the diagnosis of GDM, SBP, DBP, total cholesterol, triglyceride, HDL, LDL, GFR, ALT, AST, daily intake of calories, and weekly physical activity time (table 2).
Table 2.
Multivariable linear regression analysis results for fetuin-A associations with changes in fasting insulin, HOMA-IR, and HOMA-β from the first to the second trimester
| Change in fasting insulin | Change in HOMA-IR | Change in HOMA-β | ||||
| β | P value | β | P value | β | P value | |
| Fetuin-A in the first trimester | −0.005 | 0.0049 | −0.001 | 0.142 | −0.153 | 0.008 |
| Change in fetuin-A from the first to the second trimester | 0.032 | <0.001 | 0.007 | <0.001 | 0.426 | 0.001 |
Adjusted for maternal age, pre-pregnancy body mass index (BMI), gestational weight gain (GWG) before oral glucose tolerance test (OGTT), gestational age at enrollment, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), glomerular filtration rate (GFR), alanine transaminase (ALT), aspartate aminotransferase (AST), daily intake of calories, and weekly physical activity time.
HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance.
Then, the study population was categorized into quartiles according to the distribution of fetuin-A concentration among controls in the first trimester, second trimester, and change from the first to the second trimester. Compared with the lowest quartile, the highest quartile revealed a higher proportion of patients with GDM (table 3). In multivariate logistic regression analysis, compared with the lowest quartile, subjects in the highest quartile of fetuin-A concentration in the first and the second trimesters were at 1.65 times (OR 2.65; 95% CI 1.27 to 5.49) and 2.77 times (OR 3.77; 95% CI 1.75 to 8.13) higher risk of developing GDM, respectively, after adjustment for potential confounders. Further adjusted for the concentration of fetuin-A in the first trimester, the highest quartile of the increase in fetuin-A concentration from the first to the second trimester was associated with a higher risk of developing GDM compared with the lowest quartile (OR 2.14; 95% CI 1.05 to 4.37, table 3). Furthermore, based on the results of ROC curves (figure 2), the optimal cut-off value of fetuin-A levels in the first trimester as an indicator for diagnosing GDM was 305.9 pg/mL, which yielded a sensitivity of 0.644 and a specificity of 0.585, with the AUC at 0.612 (95% CI 0.544 to 0.680).
Table 3.
Influence of fetuin-A on gestational diabetes mellitus
| Case, n (%) | OR (95% CI) | P value | |
| Quartiles of fetuin-A in the first trimester (range, pg/mL)* | |||
| Q1 (<193.1) | 25 (42.4) | Ref | – |
| Q2 (193.1–273.4) | 19 (35.9) | 0.84 (0.37 to 1.92) | 0.051 |
| Q3 (273.5–499.9) | 35 (51.5) | 1.76 (0.81 to 3.82) | 0.346 |
| Q4 (>499.9) | 56 (62.2) | 2.65 (1.27 to 5.49) | 0.004 |
| Quartiles of fetuin-A in the second trimester (range, pg/mL)* | |||
| Q1 (<194.1) | 20 (37.7) | Ref | – |
| Q2 (194.1–290.8) | 18 (34.6) | 1.05 (0.44 to 2.49) | 0.072 |
| Q3 (290.9–501.2) | 37 (51.4) | 2.19 (0.98 to 4.92) | 0.309 |
| Q4 (>501.2) | 60 (64.5) | 3.77 (1.75 to 8.13) | <0.001 |
| Quartiles of the change of fetuin-A from the first to the second trimester (range, pg/mL)† | |||
| Q1 (<−13.7) | 33 (50.0) | Ref | – |
| Q2 (−13.7 to 8.9) | 20 (36.4) | 0.63 (0.27 to1.46) | 0.055 |
| Q3 (9.0–29.1) | 20 (37.0) | 0.88 (0.38 to 2.05) | 0.523 |
| Q4 (>29.1) | 62 (65.3) | 2.14 (1.05 to 4.37) | 0.002 |
*Adjusted for maternal age, pre-pregnancy body mass index (BMI), gestational weight gain (GWG) before oral glucose tolerance test (OGTT), gestational age at enrollment, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), glomerular filtration rate (GFR), alanine transaminase (ALT), aspartate aminotransferase (AST), daily intake of calories, and weekly physical activity time.
†Adjusted more for fetuin-A in the first trimester.
Figure 2.
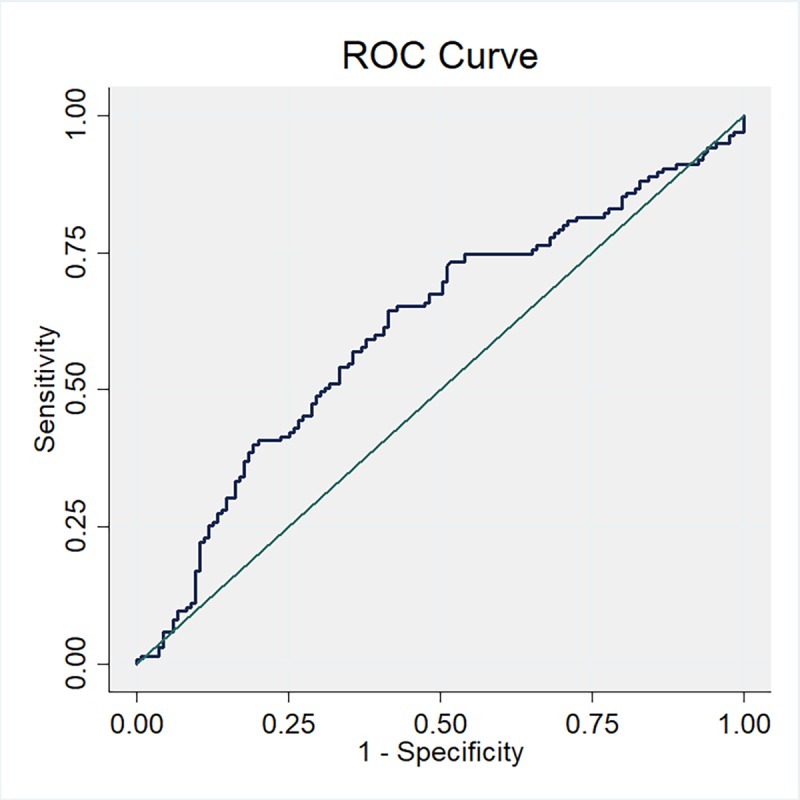
Receiver operating characteristic (ROC) curves to assess the accuracy of fetuin-A levels in the first trimester to predict gestational diabetes mellitus (GDM).
Discussion
To the best of our knowledge, this is the first study to assess the impact of the dynamic change in fetuin-A levels from the first to the second trimester on GDM, after adjustment for potential confounders, including GWG before OGTT, daily intake of calories, physical activity, and so on. First, our results showed that at both the first and the second trimesters, plasma fetuin-A levels were higher in women with GDM as compared with NGT controls, and increased significantly from the first to the second trimester. Second, the dynamic change in fetuin-A levels was associated with the changes in insulin sensitivity and β-cell function from the first to the second trimester. Third, the fetuin-A concentration in the first and the second trimesters, and its change before the diagnosis of GDM were associated with an increased risk of GDM. These findings support that fetuin-A may contribute to the pathogenesis of GDM.
In our study, we found women with GDM had significantly higher fetuin-A levels in both the first and the second trimesters, which is consistent with some previous studies. Kalabay et al 13 reported elevated fetuin-A levels in 30 patients with GDM in the 20th–40th gestational weeks compared with 30 non-pregnant controls and healthy pregnant women at any trimester (35 in the first, 31 in the second, and 38 in the third trimester). Iyidir et al 12 also found that fetuin-A concentration between 24th and 28th gestational weeks significantly increased in 26 women with GDM compared with 24 healthy pregnant women. However, Kralisch et al,15 Simjak et al,16 and Farhan et al 11 found that fetuin-A levels did not differ between women with GDM and without at 28th, 29th and 28th–38th gestational weeks, respectively. Another previous study evaluated fetuin-A levels in the first trimester (11–14 gestational weeks), and lower fetuin-A levels were found in 29 women with GDM as compared with 59 healthy pregnant women.14 Limitations of these studies included cross-sectional evaluation at a single time point before the diagnosis of GDM and modest sample sizes. Besides, different population characteristics (such as ethnicities) could partly explain the conflicting results. Furthermore, considering fetuin-A levels change dynamically during the entire pregnancy, different gestational weeks for blood sample collection could contribute to the inconsistency. In our study, case and control groups were matched for the timing of the diagnosis of GDM, ensuring the reliability of our results.
Fetuin-A might be involved in the pathogenesis of GDM by begetting insulin resistance. On the one hand, fetuin-A could inhibit insulin receptor tyrosine kinase and receptor autophosphorylation, thereby impeding the downstream signal transmission.7 On the other hand, fetuin-A is an endogenous ligand for Toll-like receptor 4, through which lipids stimulate adipose tissue inflammation, resulting in insulin resistance.21 In our study, we found the elevated fetuin-A levels were associated with aggravated insulin resistance before the diagnosis of GDM. Another prospective cohort study also showed that increasing concentration of fetuin-A was accompanied by worsening insulin sensitivity from 1 to 3 years post partum.22 Besides, an intervention study done by Malin et al showed that fetuin-A decreased by approximately 8% after 12-week exercise and lower fetuin-A after exercise was correlated with lower hepatic insulin resistance.23 These studies indicated that the levels of fetuin-A could reflect the severity of insulin resistance, and lower fetuin-A concentration might contribute to increased insulin sensitivity. Further studies are needed to clarify the biological mechanisms underlying the relationship between fetuin-A and insulin resistance. We also found the change in fetuin-A was associated with the changes in insulin concentration and HOMA-β. The possible explanation is, during pregnancy, an elevation in insulin secretion and an adaptive increase in β-cell numbers are needed to compensate for the increased insulin resistance to maintain normoglycemia.24
Another main finding of the current study was that plasma fetuin-A concentration was associated with an increased risk for the development of GDM. Of note, after adjustment for fetuin-A concentration in the first trimester, the change in fetuin-A levels from the first to the second trimester was an independent risk factor for GDM, which indicated the importance of monitoring dynamic change in fetuin-A levels during pregnancy.
The following limitations of our study must be considered. First, considering insulin resistance and β-cell function varied with ethnicity,24 the inclusion of Chinese women in our study may limit the generalizability of our results to other populations. Second, causal inferences cannot be made based on the findings of the current study because of the nature of the observational study design.
In conclusion, women with GDM had higher plasma fetuin-A levels in both the first and the second trimesters. The dynamic change in fetuin-A levels was first found to be associated with the changes in insulin sensitivity and β-cell function from the first to the second trimester, and associated with an increased risk of the development of GDM. The current findings indicated that fetuin-A could be a biomarker to predict the risk of GDM. Further studies are needed to explore the possible underlying mechanisms.
Acknowledgments
We sincerely thank the staff in Tongzhou Maternal and Child Health Hospital for data collection.
Footnotes
Correction notice: This article has been corrected since it was published. A co-corresponding author (Jue Liu) has been added.
Contributors: CYJ, HJW and JL contributed to all aspects of the study design. NH and ZLZ coordinated the data collection. CYJ performed the data analysis and drafted the manuscript with input from LZL, ZL, SSL and XRX. All authors were involved in interpreting the data and critically reviewing the manuscript drafts. All authors gave approval for the final version of the manuscript. HJW and JL are the guarantors of this work and they had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This research was funded by the National Natural Science Foundation of China (grant number 81703240 and 81973053).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the institutional review boards at Peking University (IRB00001052-18003).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data sets and deidentified participant data generated and analyzed during the current study are available from the corresponding author (HJW; ORCID identifier: 0000-0003-0849-2903) upon reasonable request.
References
- 1. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 2. International Diabetes Federation IDF diabetes atlas. 8th edn Brussels, Belgium, 2017. [Google Scholar]
- 3. Gao C, Sun X, Lu L, et al. . Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig 2019;10:154–62. 10.1111/jdi.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johns EC, Denison FC, Norman JE, et al. . Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab 2018;29:743–54. 10.1016/j.tem.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 5. Trepanowski JF, Mey J, Varady KA. Fetuin-A: a novel link between obesity and related complications. Int J Obes 2015;39:734–41. 10.1038/ijo.2014.203 [DOI] [PubMed] [Google Scholar]
- 6. Srinivas PR, Wagner AS, Reddy LV, et al. . Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 1993;7:1445–55. 10.1210/mend.7.11.7906861 [DOI] [PubMed] [Google Scholar]
- 7. Auberger P, Falquerho L, Contreres JO, et al. . Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 1989;58:631–40. 10.1016/0092-8674(89)90098-6 [DOI] [PubMed] [Google Scholar]
- 8. Mathews ST, Singh GP, Ranalletta M, et al. . Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 2002;51:2450–8. 10.2337/diabetes.51.8.2450 [DOI] [PubMed] [Google Scholar]
- 9. Guo VY, Cao B, Cai C, et al. . Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol 2018;55:87–98. 10.1007/s00592-017-1068-9 [DOI] [PubMed] [Google Scholar]
- 10. Bellamy L, Casas J-P, Hingorani AD, et al. . Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–9. 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 11. Farhan S, Handisurya A, Todoric J, et al. . Fetuin-A characteristics during and after pregnancy: result from a case control pilot study. Int J Endocrinol 2012;2012:1–5. 10.1155/2012/896736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iyidir OT, Degertekin CK, Yilmaz BA, et al. . Serum levels of fetuin a are increased in women with gestational diabetes mellitus. Arch Gynecol Obstet 2015;291:933–7. 10.1007/s00404-014-3490-3 [DOI] [PubMed] [Google Scholar]
- 13. Kalabay L, Cseh K, Pajor A, et al. . Correlation of maternal serum fetuin/alpha2-HS-glycoprotein concentration with maternal insulin resistance and anthropometric parameters of neonates in normal pregnancy and gestational diabetes. Eur J Endocrinol 2002;147:243–8. 10.1530/eje.0.1470243 [DOI] [PubMed] [Google Scholar]
- 14. Kansu-Celik H, Ozgu-Erdinc AS, Kisa B, et al. . Prediction of gestational diabetes mellitus in the first trimester: comparison of maternal fetuin-A, N-terminal proatrial natriuretic peptide, high-sensitivity C-reactive protein, and fasting glucose levels. Arch Endocrinol Metab 2019;63:121–7. 10.20945/2359-3997000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kralisch S, Hoffmann A, Lössner U, et al. . Regulation of the novel adipokines/ hepatokines fetuin A and fetuin B in gestational diabetes mellitus. Metabolism 2017;68:88–94. 10.1016/j.metabol.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 16. Šimják P, Cinkajzlová A, Anderlová K, et al. . Changes in plasma concentrations and mRNA expression of hepatokines fetuin a, fetuin B and FGF21 in physiological pregnancy and gestational diabetes mellitus. Physiol Res 2018;67:S531–42. 10.33549/physiolres.934017 [DOI] [PubMed] [Google Scholar]
- 17. Craig CL, Marshall AL, Sjöström M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 18. Ma Y-C, Zuo L, Chen J-H, Luo Q, et al. . Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937–44. 10.1681/ASN.2006040368 [DOI] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, et al. . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 20. Weinert LS. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the International association of diabetes and pregnancy study groups consensus panel. Diabetes Care 2010;33:e97 author reply e98 10.2337/dc10-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pal D, Dasgupta S, Kundu R, et al. . Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 2012;18:1279–85. 10.1038/nm.2851 [DOI] [PubMed] [Google Scholar]
- 22. Pinnaduwage L, Ye C, Hanley AJ, et al. . Changes over time in hepatic markers predict changes in insulin sensitivity, β-cell function, and glycemia. J Clin Endocrinol Metab 2018;103:2651–9. 10.1210/jc.2018-00306 [DOI] [PubMed] [Google Scholar]
- 23. Malin SK, del Rincon JP, Huang H, et al. . Exercise-Induced lowering of fetuin-A may increase hepatic insulin sensitivity. Med Sci Sports Exerc 2014;46:2085–90. 10.1249/MSS.0000000000000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mørkrid K, Jenum AK, Sletner L, et al. . Failure to increase insulin secretory capacity during pregnancy-induced insulin resistance is associated with ethnicity and gestational diabetes. Eur J Endocrinol 2012;167:579–88. 10.1530/EJE-12-0452 [DOI] [PubMed] [Google Scholar]