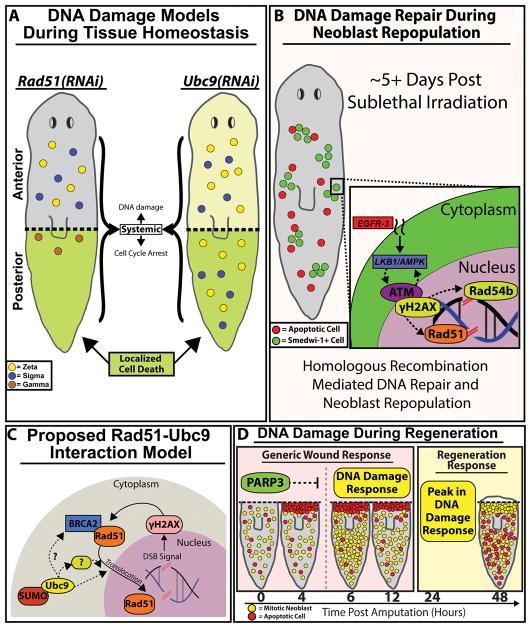
Figure 7. DNA damage repair (DDR) pathways are critical components of the cell proliferation required for tissue regeneration.
(A) Rad51(RNAi)(gray) and Ubc9(RNAi) (light-yellow) planaria share similar increases in DNA damage along the AP axis accompanied by varying levels of cell cycle arrest. Localized cell death (green) is observed in the posterior region of both RNAi conditions. Survival of neoblasts/sub-types were determined from gene expression data from figure 5G; neoblast subtypes: zeta (yellow), sigma (blue) and gamma (brown). (B) 5 days post sublethal irradiation, remaining neoblasts will repair DSBs by homologous recombination and neoblasts will repopulate the entire animal. We propose a hypothetical model whereby EGFR signaling integrates with DDR to facilitate neoblast repopulation post-irradiation (indicated with dotted lines). (C) Proposed RAD51-UBC9 interaction model. Presence of DNA DSB breaks triggers upregulation of RAD51 and γ-H2AX that together participate in DNA repair. However, after Ubc9(RNAi) the RAD51 protein accumulates in the cytoplasm as SUMOylation is required for RAD51 translocation to the nucleus. We propose that Ubc9 may interact with intermediate regulators of Rad51 for DDR. (D) Model of DNA damage during large-scale tissue regeneration. PARP3 expression peaks during the first 4hpa and may be suppressing DDR gene expression within that timeframe. Upon the decline of PARP3 expression, DDR expression at +6hpa is observed. A substantial peak in DDR observed in parallel with the regeneration responses that begins +24hpa. The robust responses of DDR pathways during wound repair could be necessary to drive DNA repair during neoblast proliferation that is required in tissue regeneration.